

25. Environmental aspects of compounds |
1179 |
H ONO2
NO3 +
H ONO2
+ NO2  (including nitronaphthalenes)
(including nitronaphthalenes)
FIGURE 6. Reaction sequence for PAH reaction with N2O5 NO3 NO2 system
TABLE 3. Calculated atmospheric lifetimes of PAH due to gas-phase reactions with OH and NO3 radicals, O3 and N2O547
|
|
|
|
Lifetime due to reaction with |
|
|
|
|
|||||
PAH |
|
OHa |
NO3b |
N2O5c |
O3d |
||||||||
Naphthalene |
8.6 |
h |
|
|
|
83 |
days |
>80 days |
|||||
|
|
|
|||||||||||
1-Methylnaphthalene |
3.5 |
h |
|
|
|
35 |
days |
>125 |
days |
||||
|
|
|
|||||||||||
2-Methylnaphthalene |
3.6 |
h |
|
|
|
28 |
days |
>40 days |
|||||
|
|
|
|||||||||||
Acenaphthylene |
1.7 |
h |
13 min |
|
|
|
¾43 min |
||||||
|
|
|
|||||||||||
Acenapthene |
1.8 |
h |
2.5 h |
21 |
days |
>30 |
|
days |
|||||
Biphenyl |
2.1 |
days |
|
|
|
>16 yr |
>80 |
|
days |
||||
|
|
|
|||||||||||
Phenanthrene |
6.0 |
h |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Anthracene |
1.4 |
h |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Fluoranthene |
3.7 |
h |
|
|
|
64 |
days |
|
|
|
|
||
|
|
|
|
|
|
|
|||||||
Pyrene |
3.7 |
h |
|
|
|
21 |
days |
|
|
|
|
||
|
|
|
|
|
|
|
|||||||
Acephenanthrylene |
1.8 |
h |
2.5 h |
21 |
days |
>30 |
|
days |
|||||
a For a 12-h daytime OH radical concentration of 1.5 ð 106 molecules cm 3.
b For a 12-h nighttime NO3 radical concentration of 2.4 ð 108 molecules cm 3.
c For a 12-h nighttime N2O5 radical concentration of 2.0 ð 1010 molecules cm 3. d For a 24-h daytime O3 radical concentration of 7 ð 1011 molecules cm 3.
The soot particles emitted from the diesel and petrol exhaust were found to be very stable over a period of hours. These PAH could undergo nitrosation with nitrogen oxides also produced in the combustion process to give rise to nitroarenes. Diesel particles obtained from a car engine were reacted with N2O5 and their rate constant was determined. The rate of reaction for the degradation of particulate PAH on atmospheric soot in presence of gas-phase N2O5 was given by the expression50:
r D kspec[PAH]mass[O]
where r is the rate of reaction in moles/unit time and kspec is the specific composite constant unique to the particle size distribution; [PAH]mass denotes the surface coverage and [O] is the concentration (mass/volume) of the gas oxidant (i.e. N2O5). The rate constants for N2O5 on atmospheric soot particles were found to be in the range from 5 ð 10 18 to 3 ð 10 18 cm 1 molecule 1 s 1.

1180 |
H. K. Chagger and A. Williams |
Similarly, the rate constants for gas-phase PAH and N2O5 were determined from the following expression:
rate D kg[N2O5] [PAH]
where the rate equals the gas-phase rate constant kg multiplied by the vapour-phase concentrations of [N2O5] and [PAH]. The rate constants reported for N2O5 with methylnaphthalenes are in the range of 1.4 ð 10 17 to 5.7 ð 10 17 cm3 molecule 1 s 1. It was also estimated that nearly 95% of gaseous PAH would react with N2O5 to give nitro-PAH.
Most PAH are carcinogenic in nature. The biological effects can be put into two categories; effects on health and effects on the ecosystem. Both can be acute or on a long term basis. The different biological responses can be related to each other since the same substance can give rise to several reactions in the organism or in the ecosystem. Nitro-PAH are considered to be even more potent carcinogens than their parent molecules. Hence, nitro-PAH can be classified as possible etiologic agents. Present data do not demonstrate a convincing association between exposure of nitro-PAH found in the petrol and diesel exhaust to that of lung cancer incidence. However, inhalation studies of structures analogous to that of benzo[a]pyrene (1,6-dinitropyrene, 6-nitrochrysene) have shown these to be tumorigenic to rodent lung51. The structures are shown in Figure 7. However, an epidemilogic study of motor exhaust-related occupation has suggested a possible risk for bladder cancer. Both 2-nitronaphthalene and 4-nitrobiphenyl have been attributed to induce bladder tumours, although bioassay data are limited52.
Application of oxygen enrichers in diesel engines, which are made of an assembly of a large number of hollow fibers, has been shown to emit low levels of soot and NOx . The oxygen enrichers can dissolve oxygen from the atmospheric air and this technique can keep a balance between the air/fuel ratio. The exhaust gas containing the polyaromatics is recycled into the engine and subsequently oxidized into leading to low emissions of soot and NOx .
Table 4 gives a list of nitro-PAH arising directly from combustion measured in different cities of the world. This is presented as air concentration and as nitro-PAH levels in soot.
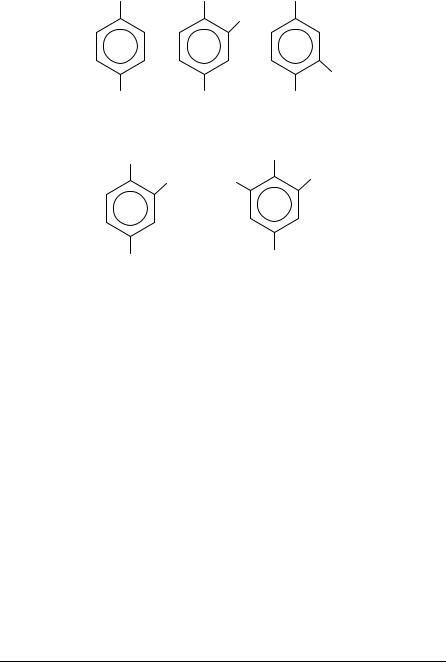
Nitrated phenols were identified in fog water in north-eastern Bavaria54. Phenols are emitted mainly through combustion processes55 or evaporation from the waste water and are very reactive in the atmosphere. The nitrated phenols are formed by atmospheric photochemical reactions of aromatic compounds such as benzene, toluene and cresols with OH-radicals and nitrogen oxides56 59. Figure 8 shows the structure of nitrophenols identified in the fog. Some of these nitrophenols are included by the Environmental Protection Agency (EPA) list as pollutants, although hardly any data are available regarding their
NO2
NO2 |
NO2 |
|
1,6-Dinitropyrene |
6-Nitrochrysene |
Benzo[a]pyrene |
FIGURE 7. Nitroarene structures analogous to that of benzo(a)pyrene
25. Environmental aspects of compounds |
1181 |
|||
OH |
|
OH |
OH |
|
|
|
NO2 |
|
|
|
|
|
|
CH3 |
NO2 |
|
NO2 |
NO2 |
|
4-Nitrophenol |
2,4-Dinitrophenol |
3-Me-4-NP |
|
|
OH |
|
|
OH |
|
|
|
|
|
|
|
CH3 |
H3 C |
NO2 |
|
|
|
|
||
|
NO2 |
|
NO2 |
|
|
|
|
|
|
|
|
|
||||||
|
2-Me-4-NP |
DNOC |
|
|
|
|
|
|
|
|
|
|||||||
FIGURE 8. Nitrophenols identified in the fog |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TABLE 4. Occurrence of nitroarenes53 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chemical (mutagenicity) |
Location/Source |
ng/m3 air |
mg/g extract from soot |
|||||||||||||||
3-Nitrotoluene |
Ambient air |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Boise, ID U.S. |
|
|
|
|
|
|
|
|
0.1 |
|
|
|
0.6 |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
1-Nitronaphthalene |
Diesel particles |
|
|
|
|
|
|
|
|
0.3 |
|
|
|
0.7 |
||||
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
Ambient air |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Los Angeles, CA |
2 |
|
3 |
|
|
0.4 |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Boise, ID |
0.03 |
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
3-Nitrobiphenyl |
Ambient air |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Los Angeles, CA |
0.03 |
|
|
0.1 |
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
Boise, ID |
0.6 |
|
|
6.0 |
|
|
|
|
|
186 |
|||||||
|
|
|
|
|
|
|
|
|||||||||||
2-Nitrofluorene |
Diesel emissions |
|
|
|
|
|
|
|
|
71 |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Air particles |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tokyo, Japan |
ND |
|
|
22 |
ND |
|
|
0.3 |
|||||||||
|
|
|
|
|||||||||||||||
|
China |
0.03 |
|
0.7 |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
||||||||
9-Nitroanthracene |
Germany |
0.2 |
|
|
5 |
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diesel |
|
|
|
|
|
|
|
|
5 |
|
94 |
||||||
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Ambient air |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Los Angeles, CA |
0.05 |
|
0.1 |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
Boise, ID |
0.04 |
|
1.5 |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
Columbus, OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outdoors |
0.01 |
|
0.1 |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
Indoors |
0.04 |
|
1.3 |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
||||||||
(continued overleaf )

1182 |
H. K. Chagger and A. Williams |
|
|
|
|
|
|
|
|
|
|
||||||||||||
TABLE 4. (continued) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Chemical (mutagenicity) |
Location/Source |
ng/m3 |
|
air |
mg/g extract from soot |
||||||||||||||||||
1-Nitropyrene |
Diesel particles |
|
|
|
|
|
|
|
|
100 |
|
|
|
|
200 |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
Gasoline particles |
|
|
|
|
|
|
|
|
2.5 |
|
|
|
|
|
|
|
|
|
||||
|
Air particles |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Detroit, MI |
0.2 |
|
|
0.6 |
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Tokyo, Japan |
0.2 |
|
|
1.6 |
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Boise, ID |
0.06 |
|
|
|
0.1 |
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
Los Angeles, CA |
0.03 |
|
|
|
0.04 |
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
Columbus, OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Outdoors |
|
|
|
|
|
|
|
|
0.01 |
|
|
|
0.05 |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
Indoors |
|
|
|
|
|
|
|
|
0.005 |
|
0.1 |
|||||||||||
|
|
|
|
|
|
|
|
|
|
||||||||||||||
2-Nitrofluroanthene |
Air particles |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Boise, ID |
0.07 |
|
|
|
0.2 |
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
Los Angeles, LA |
0.3 |
|
|
0.4 |
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Columbus, OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Outdoors |
0.03 |
|
|
|
0.2 |
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
Indoors |
0.01 |
|
|
|
0.2 |
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
3-Nitrofluoranthene |
Air particles |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Boise, ID |
0.07 |
|
|
|
0.2 |
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
Los Angeles, LA |
0.3 |
|
|
0.4 |
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Columbus, OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Outdoor |
0.03 |
|
|
|
0.2 |
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
Indoors |
0.01 |
|
|
|
0.2 |
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
3-Nitrofluoranthene |
Diesel particles |
0.9 |
|
|
7.0 |
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
6-Nitrobenzo(a)pyrene |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diesel particles |
ND |
|
|
50 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Gasoline particles |
0.2 |
|
|
33 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Air particles |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michigan, U.S. |
0.9 |
|
|
2.5 |
0.04 |
|
|
|
0.3 |
|
|
|
|
|
1.6 |
||||||||
|
|
|
|
|
|
|
|
|
|
||||||||||||||
1,3-Dinitropyrene |
Diesel particles |
|
|
|
|
|
|
|
|
ND |
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
Kerosene heater |
|
|
|
|
|
|
|
|
0.5 |
|
|
|
|
|
|
|
|
|
||||
|
Air particles |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Tokyo, Japan |
|
|
|
|
|
|
|
|
0.005 |
|
|
|
||||||||||
1,6-Dinitropyrene |
Diesel particles |
ND |
|
|
1.2 |
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
Air particles |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Michigan, U.S.. |
0.004 |
|
|
0.05 |
0.1 |
|
|
|
4.4 |
|||||||||||||
|
|
|
|
|
|
||||||||||||||||||
|
Tokyo, Japan |
0.005 |
|
|
0.1 |
0.3 |
|
|
|
8.7 |
|||||||||||||
|
|
|
|
|
|
||||||||||||||||||
1,8-Dinitropyrene |
Diesel particles |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Air particles |
|
|
|
|
|
|
|
|
ND |
|
|
3.4 |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
Michigan US |
0.04 |
|
|
|
3.8 |
0.002 |
|
0.5 |
||||||||||||||
|
|
|
|
|
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
concentrations and fluxes. Nitrophenols have been suggested to be possible contributors to forest decline60.
B. Nitroso Compounds
For the past three decades, active research has been carried out on N-nitroso compounds (NOC) and related compounds. The impact of NOC and nitrogen oxides regarding their effect on human health and safety aspects is well documented in the literature61 63. Exposure to nitrogenous chemicals, pollutants and their precursors is mainly via ingestion; however, in some cases inhalation is the major route of exposure.
The nitroso compounds can be formed by reaction with organic compounds present in the atmosphere and include two categories namely N-nitroso and C-nitroso compounds.

25. Environmental aspects of compounds |
1183 |
The former are formed by the reaction of aromatic amines and NOx in the atmosphere whereas the latter are formed during combustion processes. N-nitroso compounds have been studied extensively and some of them are discussed below.
The N-nitroso compounds are substances that have a characteristic linkage of a secondary nitrogen atom to the nitroso group, NDO. These compounds can be formed by interaction of nitrosable substrates with nitrosating agents as illustrated in Figure 9.
Nitrosamines, which are the amides of nitrous acid, are more stable and are derived from secondary amines with nitrous acid. N-nitrosamides are substances which have a carbonyl group attached to a nitrogen-bearing NO group, e.g. N-nitrosamides, N-nitrosocarbamates and N-nitrosoureas; see Figure 10.
NOC are widely distributed in the human environment and their largest exposures occur in certain work environments. However, very little data are available on the occupational exposure of NOC. The general situation for occupational exposure to NOC is summarized later in Table 5.
1. Leather and tanning
In the leather and tanning industry dimethylamine sulphate is used in depilation processes. Under alkaline conditions, dimethylamine is released into the atmosphere and it reacts with nitrogen oxides produced from exhaust emissions, to give
Nitrosable |
Nitrosating agents |
|||||||
R |
R |
HNO2 |
N2 O3 |
|||||
NH |
NR |
RONO |
M |
|
|
NO |
||
R |
R |
|
|
|||||
|
||||||||
|
|
|
|
|
|
|
||
Other nitrogen compounds |
NOx |
O |
|
|
N |
|
Y |
|
|
|
|
||||||
|
|
|||||||
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
O |
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
R |
|
R |
|
|
|
|
FIGURE 9. Routes leading to formation of nitrosamines |
|
|
|
|||||||
O |
|
O |
|
O |
|
R3 |
O |
|
|
|
|
N |
|
N |
|
N |
|
N |
|
||
|
|
|
|
|
|
|||||
|
N |
|
N |
R3 |
N |
N |
|
N |
O |
|
R1 |
R1 |
R1 |
R1 |
|||||||
R2 |
|
|
R3 |
|
R2 |
|||||
|
|
|
O |
|
|
O |
|
|
O |
|
Nitrosamine |
|
N-Nitrosamide |
|
N-Nitrosourea |
N-Nitrosocarbamate |
|||||
R1, R2 = alkyl, aryl;R3 = H, alkyl, aryl
FIGURE 10. Structure of N-nitrosamines and N-nitrosamides
1184 |
H. K. Chagger and A. Williams |
N-nitrosodimethylamine (NDMA). N-Nitrosomorpholine (NMOR) is also produced in this process, but the origin of this pollutant is unknown. Samples collected from different tanneries showed airborne nitrosamine contamination ranging from 0.05 47 mg/m3 NDMA (mean 3.4 mg/m3) and 0.05 2.0 mg/m3 NMOR (mean 0.2 mg/m3 64. Studies have indicated the possible risk of nasal cancer to workers exposed to NDMA at a daily exposure level of 440 mg NDMA/person/day and 20 mg NMOR/person/day65. Animals exposed to long-term inhalation of NDMA were found to have formed malignant tumours of mainly the liver and kidney66.
2. Rubber industry
The formation of nitroamines occurs due to the use of certain vulcanisation accelerators such as thiurams, dithiocarbamates and sulphenamides. These agents are nitrosated during the vulcanisation process. The origin of the NOC is primarily due to the adsorption of NOx on the large surface of inorganic rubber additives, e.g. zinc oxide and carbon black or nitrosating rubber chemicals. Figure 11 shows the nitrosation reactions of typical accelerators67.
The extent of formation of these NOC depends upon the presence of nitrogen oxides present in the atmosphere during the manufacturing cycle. The major contaminants are NDMA, N-nitrosodiethylamine (NDEA), N-nitrosopyrrolidine (NPYR), NMOR, N- nitrosodiphenylamine (NDPhA), N-nitrosopiperidine (NPIP) and N-nitrosodibutylamine (NDBA)68. NMOR was found in the hot process areas; NDMA occurred in tube production areas in which NDPhA was being used as retarder and tetramethylthiuram disulphide as an accelerator. Figure 12 shows a proposed reaction scheme of formation of NOC in the rubber industry and subsequent exposure67.
The nitrosamine formation can be controlled by meeting the following regulations69:
žBlock or reduce nitrogen oxide species, have adequate ventilation
žDegrade nitrosamine
žUse amine-free accelerators by changing compounds
CH3 |
S |
|
|
|
|
S |
CH3 |
|
|
CH3 |
|
|
|
|
|
|
|
|
|
||||
N |
C |
S S |
|
C |
N |
ON |
N |
NDMA |
|||
CH3 |
|
|
|
|
|
|
CH3 |
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S |
|
|
|
|
|
C4 H9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Zn S |
C |
N |
|
|
|
ON |
N |
NDBA |
|
|
|
|
|
|
|
||||||||
|
|
2 |
|
|
|
|
C4 H9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
S |
|
|
|
|
|
|
|
|
|
|
Zn S |
C |
N |
|
|
ON |
N |
NPIP |
|
|
||
|
|
|
|
||||||||
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
||
|
|
|
|
S |
|
N |
O |
|
ON |
N |
O NMOR |
|
|
|
|
|
|
||||||
|
|
S |
|
|
|
|
|
|
|
||
FIGURE 11. Formation of different NOC from their corresponding accelerators
25. Environmental aspects of compounds |
1185 |
|||
|
Use of amine precursors |
|
|
|
|
in industry |
|
|
|
Process |
Use of NOx-releasing |
|
||
(heat) |
Chemicals |
|
||
Release of amines |
Nitrosamine formation |
|
||
|
|
in the product |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
Nitrosamine formation |
Degassing of nitrosamines |
|
in the air |
||
|
Exposure to operators
FIGURE 12. Proposed reaction mechanism of formation and exposure
3. Metal and machining
One of the major pollutants in this industry is N-nitrosodiethanolamine (NDELA) arising from cutting fluids. The simultaneous use of diethanolamine or triethanolamine cutting fluids with nitrite as an anticorrosion (antioxidative) agent in the formulation results in NDELA production. Workers come into direct contact due to inhalation of oil mists as they handle the products directly. The demonstration of dermal penetration of NDELA has been shown in both humans and animals. NDELA in laboratory animals has been shown to induce cancer in different organs like liver, kidneys, nasal cavity and papilloma of trachea70,71. Workers staying in rooms with 1 mg/m3 revealed two times more DNA damage in mononuclear blood cells than those staying in an environment with less than 50 ng/m3. However, no significant correlation was obtained between the extent of DNA damage and the extent of skin contact or the concentration of NDELA found in the cutting fluids72.
Table 5 gives the exposure level of different N-nitrosamines analogously arising as pollutants from various chemical industries73.
TABLE 5. Occupational exposure to N-nitroso compounds
Industry/occupation |
N-Nitrosamine |
Exposure levels |
|
|
|
Metal working industry |
N-nitrosodiethanolamine (NDELA) |
>50 |
|
N-nitrosodiethanolamine (NDELA) |
>50 |
Metal foundries (core-making) |
N-nitrosodimethylamine (NDMA) |
>5 |
|
N-nitrosodiethylamine (NDEA) |
>5 |
Leather tanneries |
N-nitrosodimethylamine (NDMA) |
>50 |
Rubber and tyre industry |
N-nitrosodimethylamine (NDMA) |
>50 |
|
N-nitrosodiethylamine (NDEA) |
>5 |
|
N-nitrosodibutylamine (NDBA) |
>5 |
|
N-nitrosomorpholine (NMOR) |
>50 |
|
N-nitrosomethlyphenylamine (NMPhA) |
>50 |
Chemical industries |
|
|
Rocket fuel industry |
N-nitrosodimethylamine (NDMA) |
>50 |
Dye manufacture |
N-nitrosodimethylamine (NDMA) |
<5 |
|
N-nitrosodiethylamine (NDEA) |
<5 |
Detergents and surfactants |
N-nitrosodimethylamine (NDMA) |
<5 |
Amine and pesticide production |
N-mononitrosopiperazine (NMPZ) |
<5 |
Fish processing industry |
N-nitrosodimethylamine (NDMA) |
<5 |
Warehouse and sale rooms |
N-nitrosodimethylamine (NDMA) |
>5 |
(especially for rubber products) |
N-nitrosomorpholine (NMOR) |
>5 |
|
|
|
1186 |
H. K. Chagger and A. Williams |
IV. ENVIRONMENTAL EXPOSURE TO PREFORMED NITROSAMINES
The presence of non-volatile NOC, i.e. preformed nitrosamines, has been reported in various cosmetics, pharmaceutical products, foods, beverages and dairy products.
A. Sunscreens and Cosmetics
Nitrosamine contamination of cosmetic products and toiletries may result through formulation with nitrosamine contaminated amines or via formulation by contact with nitrosating agents or bactericides. Market surveys have detected up to 45 ppm nitrosodiethanolamide and 21 ppm 2-ethylhexyl 4-(N-methyl-N-nitrosamino) benzoate in several sunscreens and cosmetic products. Oxides of nitrogen can also act as potential nitrosating agents in cosmetics. The extent of exposure depends upon the frequency of usage, degree of absorption through the skin and nitrosamine stability on exposure to UV. Products like sunscreens, after being applied to skin, leave a non-aqueous layer as the water from the emulsion evaporates. Oxides of nitrogen can readily be absorbed into a non-polar matrix and nitrosate amines to produce nitrosamines74. However, the stability of nitrosamine can be questioned as it decomposes in the presence of UV light75. Hence, more research needs to be carried out where human exposure to NMPABOA and its decomposition in sunlight is concerned, although the carcinogenecity of NMPABOA is uncertain. The products can also undergo nitrosation over a period of time depending on conditions like its storage and temperature. A sunscreen product containing 2-ethylhexyl 4-(N,N-dimethylamino) benzoate (Padimate O) purchased in 1987 was free of nitrosamine contamination. The same product was found to contain 8 ppm nitrosamine derivatives in the year 1990. Hence, seasonal products such as sunscreen, not sold by the end of the summer, may be affected by nitrosamine levels in products depending on their storage conditions.
B. Pharmaceutical Products
Several pharmaceutical products undergo nitrosation and form nitrosamines during synthesis and storage in vivo under gastric conditions in human beings. Investigations have reported the development of tumours in test animals when they were exposed to longterm concurrent nitrite and drug feeding76,77. This in turn has caused some governments to impose legislation for the removal of nitrosamines from these products before the sale. Aminophenazone, a precursor to NDMA, was shown to induce sarcoma in liver and lung and hence has been removed from some pharmaceutical markets.
The production of hydrazine and hydrazones by reduction of nitrosamines is another route for NOC production. Piperazine also leads to production of NOC and its use as an antihelminthic has decreased considerably in most developed countries, though not in the case of developing countries due to its low cost.
Two N-nitrosoureas, Bischloroethyl-nitrosourea (BCNU) and 1-chloroethyl-3- cyclohexyl-1-nitrosourea (CCNU), have been used as anticancer agents in clinics but their mechanism and their toxicities have yet to be determined78.
C. Agricultural Products
There are several routes for nitrosamine contamination in pesticides: use of contaminated chemicals during synthesis, side reactions, use of nitrite as a preservative and corrosion inhibitor of metal containers and by reactions with environmental nitrosating agents. Over 300 formulations were shown to be contaminated with nitrosamines; however, the main contamination was confined to 2,6-dinitroaniline herbicides, dimethylamino salts of phenoxyalkanoic acid herbicide, diethanolamine and triethanolamine salts of acid
25. Environmental aspects of compounds |
1187 |
pesticide, quaternary ammonium compounds and morpholine derivatives79. Presence of NDPA in herbicide trifluralin was reported due to nitrosation of the respective amine used during synthesis.
Accumulation of agricultural chemicals in soils may lead to formation of nitrosamines. The herbicides atrazine and butralin were found to form nitrosamines only in the presence of high levels of nitrite. Active uptake of NDMA and NDEA by wheat and barley has been published; however, no conclusive evidence has been reported80.
D. Packing materials
Migration of nitrosamines into consumer products can occur via direct contact of materials such as waxed containers, elastic and rubber etc.81. Morpholine is used extensively as an industrial solvent for wax formulations. The wax formulations are used for coating fruits and vegetables to prevent moisture loss and increase shelf-life of the products. Paper and cardboard packed with morpholine was also found to give rise to NDMA, as these packaging materials were found to be contaminated with NDMA as well. Besides this, rubber products also provided a migratory source for both nitrosamines and nitrosable amine precursors, as trace levels of NDEA and N-nitrosodibutylamine (NDBA) have been reported in cured meats with amine-based accelerators in the rubber nettings82.
E. Foods and beverages
NOC constitute a large category of genotoxic chemical carcinogens occurring in human diet and are known to induce cancer in experimental animals. Nitrosamines are generally found in foods since they are more stable than nitrosamides. Some NOC precursors do not act directly but must be converted to other nitrosation species.
Human exposure to nitrates is via exposure to food and drinking water. The nitrates in food may be present naturally or as an additive introduced for various technological reasons. Nitrite is added to foods for preservation, but is reactive in foods, whereas nitrate is quite unreactive.
Vegetables are also a prime source of nitrate, and variations in their nitrate levels occur due to conditions employed during the cultivation and storage processes. The nitrate concentration in surface water has increased due to increased use of artificial fertilizers, changes in land use and disposal of waste from intensive farming. Nitrate is readily converted in mammalian systems through bacterial and mammalian enzymes to nitrite which can react with amines, amides and amino acids to form NOC.
Critical analysis has shown that most dietary components contaminated with NOC can be classified into different categories as follows83:
žIn foodstuff preserved by addition of nitrate/nitrite (namely cured meat produce and cheeses) both methods of preservation introduce nitrosating species into the food matrix.
žIn foodstuffs preserved by smoking (such as fish and meat products) oxides of nitrogen present in the smoke act as nitrosating agents.
žNitrosated amino acids during cooking yield corresponding volatile nitrosamines: N- nitrosoproline (NPRO), N-nitroso-4-hydroxyproline (NHPRO) and N-nitrososarcosine (NSAR), respectively.
žConcentration of different N-nitrosamines in nitrite-cured meat products is further increased following smoking processes.
žFoodstuffs subject to drying by combustion gases (containing oxides of nitrogen) such as malt for production of beer and whiskey, low-fat dried milk products and spices.

1188 |
H. K. Chagger and A. Williams |
žPickled and salt-preserved foods, in particular plant-based products (pickled vegetables) in which microbial reduction of nitrate to nitrite occurs. Foodstuffs stored under humid conditions favouring fungal contamination, particularly the growth of Fusarium moniliforme.
žMigration and formation of nitrosamines from food contact materials.
The last source of NOC that has been a major source for health concern of infants is usage of rubber pacifiers and baby feeding bottles fitted with rubber nipples. NOC present in the rubber formulations can migrate into baby foods and drinks and into meat packed in rubber nettings.
Various NOC can be found in food processing operations. The most commonly known contributors to dietary volatile and non-volatile N-nitrosamines are nitrite cured meats, particularly fried bacon and beer. Several reviews cover the occurrence and formation of NOC in foods and beverages84 86.
The contamination in beer with NOC was first reported in Germany in 197987. The contamination of beer occurs during the kilning (drying) process of malt88 and fermentation89, which leads to the occurrence of NDMS and NPYR. The nitrogen oxides were identified as a source of nitrosamine formation in the beer, formed by nitrosation of the alkaloids present in the malt90.
Since NDMA is a potent carcinogen it could pose serious health implications, as beer is widely used in Western Europe. A recent study which involved 14 German beers could detect only two compounds, NDMA (0.17 š 0.18 mg/kg) and NPYR (1.5 š 1.01 mg/kg), in very low concentrations. Continuous efforts by the brewing industry and change in brewing technology has resulted in a significant reduction in the NDMA contamination by 1 5% over the years. See Table 6 for details. Hence it can be concluded that for moderate beer drinkers, current levels of NDMA are unlikely to represent a significant health risk, and NPYR was shown to be non-carcinogenic92.
TABLE 6. Reduction of N-nitrosodimethylamine (NDMA) in beer and some representative current data91,85
|
|
NDMA (mg/kg) |
||||||
Country |
Year |
mean |
|
range |
||||
|
|
|
|
|
|
|
|
|
FRG |
1977/78 |
2.7 |
0 |
|
68 |
|
||
|
||||||||
|
1980 |
0.28 |
0 |
|
9.2 |
|||
|
|
|||||||
|
1981 |
0.44 |
0 |
|
7.0 |
|||
|
|
|||||||
|
1989 |
0.16 |
0 |
|
1.7 |
|||
|
|
|||||||
|
1990 |
0.17 |
0 |
|
0.6 |
|||
|
|
|||||||
USA |
1980 |
5.9 |
0 |
|
14 |
0.99 |
||
|
||||||||
|
1988 |
0.26 |
0.03 |
|
||||
|
|
|||||||
Canada |
1978 |
1.4 |
0.60 |
|
4.9 |
|||
|
||||||||
|
1982 |
0.31 |
0 |
|
1.9 |
|||
|
|
|||||||
USA & Canada |
1989 |
0.07 |
0 |
|
0.58 |
|||
|
||||||||
Netherlands |
1979 |
2.0 |
0 |
|
7.4 |
|||
|
||||||||
|
1980 |
0.2 |
0 |
|
1.2 |
|||
|
|
|||||||
Italy |
1982 |
0.4 |
0 |
|
0.79 |
|||
|
||||||||
|
1986 |
0.3 |
0 |
|
0.71 |
|||
|
|
|||||||
Sweden |
1988 |
0.2 |
0 |
|
6.5 |
|||
|
||||||||
Poland |
1989 |
0.2 |
0 |
|
0.3 |
|||
|
||||||||
China |
1981 |
2.1 |
0 |
|
6.5 |
|||
|
||||||||
|
1987 |
0.5 |
0 |
|
6 |
|
|
|
|
|
|
|
|||||
Japan |
|
|
0 |
|
<5 |
|||
|
|
|
||||||
UK |
|
|
0.9 |
|
23 |
|||
|
|
|
||||||
|
|
|
|
|
|
|
|
|
