

Sudan IV
Guiba Black D
(C.I. Direct Black 17)
Methyl Red
Methyl Orange
N,N-Dimethyl-4- aminoazobenzene
N-Methyl-4- aminoazobenzene
4-Aminoazobenzene
2-Methyl-N,N dimethyl-4- aminoazobenzene
|
CH3 |
|
CH3 OH |
|
|
N N |
|
N N |
|
|
|
|
OH |
|
|
|
OCH2 |
NH3 |
|
|
|
|
|
|
H2 N |
N N |
|
N N |
|
|
H3 C |
|
NaO3 S |
|
|
|
|
|
|
|
HO |
|
|
|
|
O |
|
CH3 |
|
|
N N |
|
||
|
N |
|
||
|
|
|
CH3 |
|
|
N2 O2 S |
N N |
N(OH2 )2 |
|
|
N N |
CH3 |
|
|
|
H |
|
||
|
|
|
CH3 |
|
|
N |
N |
NHCH3 |
|
|
N N |
NH3 |
|
|
|
|
H3 C |
|
|
|
N |
N |
N(CH3 )2 |
|
C
š
C
C
C
C
C
C
1199
(continued overleaf )
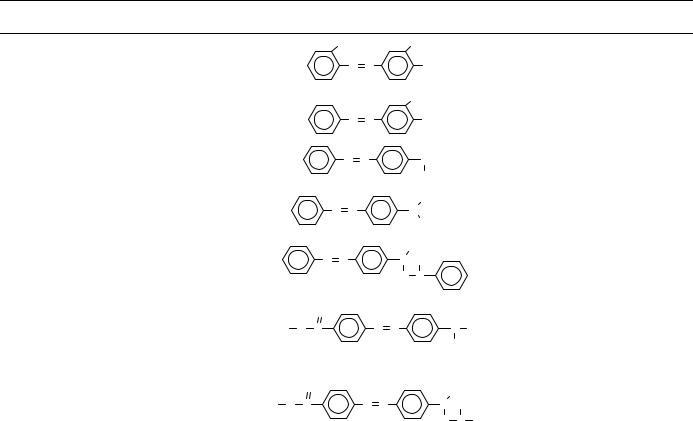
TABLE 8. (continued)
Common |
|
Chemical |
|
|
|
|
name |
|
structure |
|
|
|
|
|
|
CH3 |
|
CH3 |
|
|
O-Aminoazotoluene |
|
N |
N |
NH3 |
|
|
|
|
|
|
|||
|
|
|
|
OCH2 |
|
|
3-Methoxy-4- |
|
N N |
NH2 |
|
|
|
aminoazobenzene |
|
|
|
|||
N-Hydroxy-4- |
|
N |
N |
N(CH) |
|
|
aminoazobenzene |
|
|
|
N |
|
|
|
|
|
|
CH3 |
|
|
N-Acetoxy-N-methyl- |
|
N N |
|
N |
|
|
4-aminoazobenzene |
|
|
|
OCOCH2 |
|
|
|
|
|
|
CH3 |
|
|
N-Benzoyloxy- |
|
N N |
|
N O |
|
|
|
|
|
O C |
|
|
|
N-methyl-4- |
|
|
|
|
|
|
aminoazobenzene |
|
|
|
|
|
|
40 -Methoxycarbonyl-N- |
|
O |
|
|
|
|
CH3 |
O C |
N N |
|
H |
OH |
|
hydroxy |
|
|
|
|
CH3 |
|
N-methyl-4- |
|
|
|
|
||
|
|
|
|
|
|
|
aminoazobenzene |
|
|
|
|
|
|
40 -Methoxycarbonyl-N- |
|
O |
|
|
CH3 |
|
CH3 O |
C |
N N |
H |
O |
|
|
methoxycarbonyl |
|
|
|
O |
C |
CH3 |
N-methyl-4- |
|
|
|
|||
|
|
|
|
|
|
|
aminoazobenzene
Metabolic activationa
C
C
C
š
š
1200
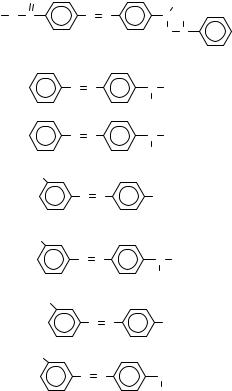
40 -Methoxycarbonyl-N- benzoyloxy- N-methyl-4- aminoazobenzene
N-Hydroxyl-N-methyl- 4-aminoazobenzene
N-Methyl-N-hydroxy- 4-aminoazobenzene
30 -Methyl-N,N dimethyl-4- aminoazobenzene
30 -Methyl-N-methyl-4- aminoazobenzene
30 -Methyl-4- aminoazobenzene
O
CH3 O C
H3 C
H3 C
H3 C
CH3
|
|
|
CH3 |
|
N N |
|
H O |
|
|
|
O C |
N |
N |
H |
CH3 |
|
|
OH |
|
N |
N |
H |
CH3 |
|
|
OH |
|
|
N N |
N(CH3 )2 |
|
N N |
H |
CH3 |
|
H |
|
N N |
NH2 |
|
š
š
š
C
C
C
30 -Methyl-N- |
N N |
C |
methyl-N-acetyl-4- |
NCH3 |
|
|
COCH3 |
|
aminoazobenzene |
|
|
|
|
|
|
|
(continued overleaf ) |
1201
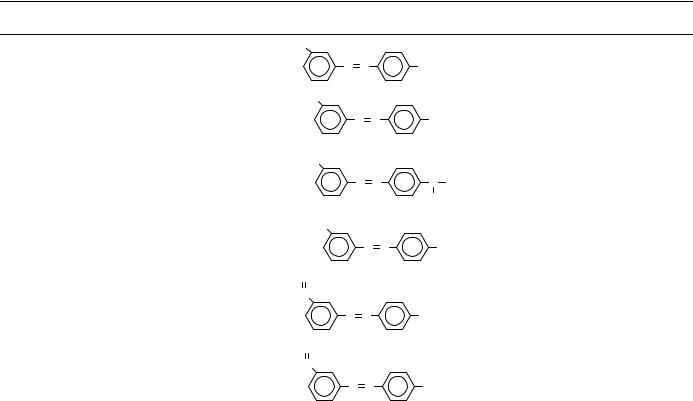
TABLE 8. (continued)
Common |
Chemical |
|
|
name |
structure |
|
|
30 -Methyl-N-acetyl-4- |
CH2 |
|
|
N N |
NHCOCH3 |
||
aminoazobenzene |
HOH2 C
30 -Hydroxylmethyl-
N,N-dimethyl-4- N N N(CH3 )2 aminoazobenzene
HOH2 C
30 -Hydroxylmethyl- N-methyl-4- aminoazobenzene
30 -Hydroxylmethyl-4- aminoazobenzene
30 -Formyl-N,N- dimethyl-4- aminoazobenzene
30 -Carboxylic N,N-dimethyl-4- aminoazobenzene
N N |
N CH3 |
|
H |
HOH2 C |
|
N N |
NH2 |
O |
|
HC |
|
N N |
N(CH3 )2 |
O
HOC
N N |
N(CH3 )2 |
1202
Metabolic activationa
C
C
C
C
C
C
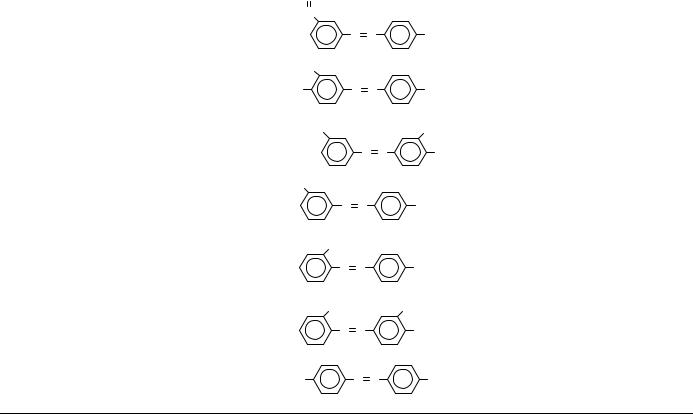
30 -Carboxylic- N-methyl-4- aminoazobenzene
30 -Methyl-40 -hydroxyl- N,N-dimethyl-4 -aminoazobenzene
3,30 -Bischloromethyl-4- aminoazobenzene
O |
|
|
|
HOC |
|
|
C |
|
|
|
|
|
N |
N |
HHCH3 |
CH2 |
|
|
C |
|
|
|
|
HO |
N |
N |
N(CH3 )2 |
CH2 Cl |
|
|
CNCH3 |
|
|
|
|
|
|
N N |
NH2 |
CH2 OAc |
|
|
|
30 -Acetoxymethane- N,N-dimethyl-4- aminoazobenzene
20 -Methyl-N,N- dimethyl-4- aminoazobenzene
20 ,3-Hydroxylmethyl- N,N-dimethyl-4- aminoazobenzene
40 -Methyl-N,N- dimethyl-4- aminoazobenzene
N |
N |
N(CH3 )2 |
C |
|
CH3 |
|
|
C |
|
N |
N |
N(CH3 )2 |
||
|
||||
CH2 OH |
CH2 OH |
C |
||
N |
N |
N(CH3 )2 |
||
|
||||
H3 C |
N N |
N(CH3 )2 |
C |
|
(continued overleaf )
1203

TABLE 8. |
(continued) |
Common |
Chemical |
name |
structure |
40 -Hydroxymethyl- N,N-dimethyl-4- aminoazobenzene
6-Dimethylaminophenyl- azobenzthiazole (6BT)
5-Dimethylamino- phenylazoindoline (5I)
Chrysoidin Y
(C.I. Index 11325)
Bismark Brown Y
(C.I. Index 21000)
Bismark Brown R
(C.I. Index 21010)
HOH2 C |
N N |
|
HN |
N |
N |
S |
|
|
HN |
N |
N |
N |
|
|
|
CH |
N2 H |
|
3 |
|
|
N N |
|
|
NH2 |
|
H2 N |
N N |
|
|
NH2 |
|
H2 N |
N N |
|
|
N(CH3 )2 |
|
|
CH3 |
|
|
N |
|
|
CH3 |
|
|
CH2 |
|
|
N |
|
|
CH2 |
|
|
NH2 |
|
|
CH3 |
|
|
H2 N |
|
N |
N |
NH2 |
|
H2 N |
|
N |
N |
NH2 |
CH3 |
|
CH3 |
CH3 |
4,40 -Diaminoazobenzene |
H2 N |
N N |
NH2 |
Metabolic activationa
C
C
C
C
C
C
C
1204

4,40 -(B-Hydroxyethyl- amino)Azobenzine
Direct Black 19
Direct Black 38
(Direct Deep Black EX)
Direct Black GB NB
|
HOH2 CH2 CHN |
N |
N |
NHCH2 CH2 OH |
C |
|
|
NH2 |
NH2 |
OH |
N2 H |
|
|
|
|
|
C |
|||
N2 H |
N N |
N N |
N N |
N N |
||
NH2 |
||||||
|
NO2 S |
SO2 N |
|
|
||
|
NH2 |
|
NH2 OH |
|
||
|
|
|
|
C |
||
H1N |
N N |
|
N N |
N N |
||
|
|
|
||||
|
|
|
NaO2 S |
SO2 Na |
|
|
|
OH |
|
|
NH2 OH |
|
|
H1N |
N N |
C NH |
N N |
N N |
C |
|
|
|
O |
HO2 S |
SO2 H |
|
|
|
|
|
|
|||
|
|
NH2 |
NH |
OH |
|
H2 N |
|
|
|
|
|
2 |
|
|
|
|
C |
Direct Black 19 |
H1N |
N N |
N N |
|
N N |
N N |
NH2 |
|
Analogue |
HO |
S |
HO S |
|
SO H |
|
SO2 H |
|
|
|
|
|
|||||
|
2 |
2 |
|
2 |
|
|
|
|
|
|
OH |
NH2 |
OH |
|
HO |
|
C |
Direct Black 19 |
OH |
N N |
N N |
|
N N |
N N |
OH |
|
Analogue |
|
|
HO2 S |
|
SO2 H |
OH |
|
|
|
HO |
|
|
|
||||
(continued overleaf )
1205

TABLE 8. (continued)
Common |
|
|
|
Chemical |
|
|
|
|
Metabolic |
name |
|
|
|
structure |
|
|
|
|
activationa |
|
HO |
OH |
|
NH2 |
OH |
|
OH |
|
C |
Direct Black 19 |
|
N N |
N N |
N N |
|
N N |
OH |
||
Analogue |
|
|
|
HO2 S |
SO2 H |
|
|
|
|
|
HO |
OH |
|
|
OH |
|
|
||
|
HOOC |
|
OH OH |
|
|
COOH |
|
||
|
|
|
|
|
|
|
|
|
|
Direct Black 19 |
HO |
N N |
|
N N |
N N |
|
N N |
HO |
C |
|
|
|
|
|
|
|
|
||
Analogue |
|
|
|
|
|
|
|
|
|
|
|
|
|
SO2 H |
|
|
|
|
|
Azo Dyes Containing the Benzidine Group |
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
H2 N SO2 Na |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H3 C |
S O |
N N |
|
N N |
|
|
š |
Brown 5 R |
|
|
O |
|
|
|
|
|
|
(C.I. Acid Orange 45) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SO2 Na |
|
|
|
|
NaO2 S |
|
SO2 Na |
NaO2 S |
|
SO2 Na |
|
|
Trypan Blue |
|
|
|
N N |
N N |
|
|
|
C |
(Direct Blue 14) |
|
NH2 |
OH |
H C |
CH |
OH |
NH2 |
|
|
|
|
|
|
||||||
|
|
|
|
3 |
3 |
|
|
|
|
|
|
NH2 |
OH |
|
|
OH |
NH2 |
|
|
|
|
NaO2 S |
|
N N |
N N |
|
SO3 Na |
|
C |
Evan’s Blue |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
||
|
|
NaO2 S |
|
H3 C |
CH3 |
|
SO3 Na |
|
|
|
|
|
|
|
|
|
|
||
1206

Congo Red
Benzopurpurin 4 B (Deltapurpurin)
Direct Blue I
Pentacyl Sky
Blue 4 B X
|
NH2 |
|
|
|
|
N N |
N N |
|
SO3 Na |
|
|
|
NH |
H3 C |
CH3 |
|
2 |
|
|
|
|
N N |
N N |
|
SO3 Na |
|
|
NH |
OH |
H3 CO |
OCH3 |
2 |
|
|
|
NaO3 S |
|
N N |
N N |
|
|
||
SO3 Na |
|
|
|
NH |
OH |
H3 CO |
OCH3 |
2 |
|
|
|
|
|
N N |
N N |
NaO2 S |
|
SO2 Na |
NaO3 S |
NH2
SO3 Na
NH2
SO3 Na
OH NH2
SO3 Na
SO3 Na
OH NH2
SO2 Na
C
C
C
C
|
|
|
O |
O |
O |
|
NaOOC |
|
|
|
C |
Direct Brown 95 |
HO |
N N |
N N |
N N |
|
|
|
|
HO |
|
SO2 Na |
|
|
|
|
|
|
|
NH2 |
OH |
|
OH |
NH2 |
Direct Blue 6 |
|
N N |
N N |
|
C |
|
NaO2 S |
SO3 Na |
NaO3 S |
|
SO3 Na |
(continued overleaf )
1207
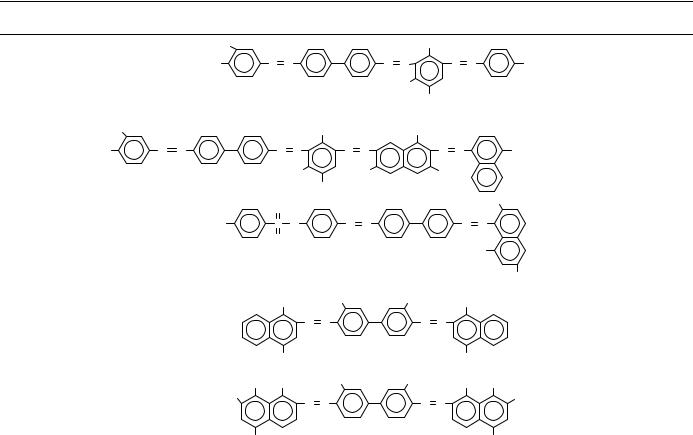
TABLE 8. (continued)
Common |
|
|
|
|
|
Chemical |
|
|
|
name |
|
|
|
|
|
structure |
|
|
|
|
NaOOC |
|
|
|
|
|
NH2 |
|
|
Direct Brown 1:2 |
HO |
N |
N |
|
|
N N |
N N |
SO2 Na |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N2 H |
|
|
|
|
|
|
|
|
|
|
OH2 |
|
|
NaOOC |
|
|
|
NH2 |
|
HO |
|
|
|
Direct Brown 31 HO |
N N |
|
N N |
|
N |
N |
N N |
SO2 Na |
|
|
|
|
N2 H |
NaO2 S |
SO2 Na |
|
|
||
|
|
|
|
OH2 |
|
|
|
HO |
|
|
|
O |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
||
|
H3 C |
S O |
|
N |
N |
N |
N |
|
|
Acid Red 85 |
|
O |
|
|
|
NaO2 S |
|
||
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
SO2 Na |
|
|
|
|
NH2 |
H3 C |
|
OH2 |
NH2 |
|
|
Direct Red 2 |
|
|
|
N N |
|
N N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SO2 Na |
|
|
SO2 Na |
|
||
|
NaO2 S |
NH2 |
OH |
H3 C |
|
OH2 |
OH |
OH |
|
|
|
|
|
|
|
|
SO |
Na |
|
Direct Blue 53 |
|
|
|
N N |
|
N N |
2 |
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
NaO2 S |
|
|
|
|
|
SO2 Na |
|
|
Metabolic activationa
C
C
C
C
C
1208
