

25. Environmental aspects of compounds |
1189 |
Over the last two decades there has been a net decrease in both nitrate and nitrite in cured meats and foods. In cured meat products it is generally accepted that the formaldehyde present in the wood smoke is involved in the formation of N-Nitrosothiazolidine (NTHZ) and N-Nitrosothiazolidine-4 carboxylic acid (NTCA). Bacon represents a unique case as uncooked bacon is generally free of nitrosamines, but high levels of nitrosamines are formed during cooking. The conditions leading to formation of nitrosamines in bacon have been widely studied and the presence of NTCA in raw and fried bacon has been reported93 97. It has been suggested that the thermal process results in transformation of NTCA into NTHZ during the frying process98.
Over 300 NOC have been shown to be carcinogenic to more than one animal species. Table 7 shows some compounds present in the diet, their occurrence and their carcinogenecity99.
F. Endogenous Formation
There is sufficient evidence to indicate that the NOC compounds represent a serious potential health hazard, although the magnitude of this hazard remains to be established. Exposure to nitroso compounds and their precursors is mainly via ingestion. However, in some cases inhalation may become the major route of exposure. Figure 13 illustrates the total exposure of NOC compounds on humans occurring via both exogenous and endogeneous routes100.
Nitric oxide is formed endogenously in the body by many types of cells for the purpose of intercellular communication (brain, cardiovascular system), or as a part of the immune or inflammatory response (macrophages, endothelial cells). The chemistry of nitric oxide formation in the body is very complex both in terms of chemical species and in the number of parallel and consecutive reactions. Damage to DNA in mammalian cells is caused by at least two major pathways: one arising from the reaction of NO with molecular oxygen and the other by reaction of NO with superoxides. The first route gives N2O3, which can either (1) nitrosate secondary amines to form carcinogenic or mutagenic N-nitrosamines or (2) nitrosate primary amines on DNA bases. The latter reaction results in deaminated bases from adenine, cytosine, 5-methylcytosine and guanine. NOC have been shown to produce cancer in humans, particularly when exposure starts early in life and persists over a long period101. Figure 14 shows endogeneous NOC synthesis in the human body102. The NOC production is brought about by bacterial enzymes which catalyse nitrosation from
Total exposure
Exogenous exposure |
Endogenous exposure |
Life style |
Occupational |
Tobacco + tobacco smoke |
Rubber industry |
Food |
Leather industry |
Cosmetic products |
Chemical industry |
Household commodities |
Mining |
Indoor air |
Pesticide production |
Drugs |
|
FIGURE 13. NOC and total exposure
Uptake of precursors |
Formation of precursors |
Nitrite |
nitrite from nitrate |
Nitrous gases NOx |
|
Nitrosatable amino |
|
compounds |
|

TABLE 7. N-Nitroso compounds in the diet, carcinogenicity and occurrence99
|
|
|
Carcinogenicity |
|
|
|
|
|
|
|
|
|
|
|
(following oral administration) |
|
Major dietary source (mg/kg) |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Concentration |
|
|
|
|
|
|
|
|
|
|
|
|
||
N-Nitroso compound |
Species |
Organotropy |
|
Foodstuff |
|
|
|
Range |
Mean |
|||
|
|
|
|
|
|
|
|
|
|
|||
N-Nitrosodimethylamine |
Rat |
Liver, kidney, (lung) |
Cured meats |
1.0 |
|
5.0 |
4.0 |
|||||
|
||||||||||||
(NDMA) |
Mouse |
Liver, kidney, lung |
Fried bacon |
<23 |
|
|||||||
|
Me |
Hamster |
Liver, (glandular stomach) |
Marine products: |
|
|
|
|
|
|
8.6 |
|
ON |
N |
Guinea pig |
Liver |
|
Dried fish (Japan) |
3.0 |
|
39 |
||||
|
|
|||||||||||
Rabbit |
Liver, lung |
|
Dried fish (Greenland) |
8.6 |
|
38 |
|
|||||
|
Me |
|
|
43.9 |
||||||||
|
|
|
||||||||||
|
Mink |
Liver |
|
Dried shrimps (China) |
5.4 |
|
132 |
|||||
|
|
|
|
|||||||||
|
|
|
|
|
Broiled squid (China) |
<300 |
|
|||||
|
|
|
|
Millet flour and grain |
|
|
|
|
|
|
|
|
|
|
|
|
|
products (China) |
0.1 |
|
1.3 |
0.5 |
|||
|
|
|
|
|
|
|||||||
|
|
|
|
Dairy and cheese products |
1 |
|
6 |
|
|
|||
|
|
|
|
|
|
|
||||||
|
|
|
|
Dried milk products |
<7.0 |
|
||||||
|
|
|
|
Edible oils and fats |
<1.0 |
|
||||||
|
|
|
|
Pickled/fermented vegetables |
<5.0 |
|
||||||
|
|
|
|
Beer |
<0.5 |
0.2 |
||||||
|
|
|
|
Alcoholic beverages (whisky) |
<2.0 |
1.0 |
||||||
N-Nitrosoethylmethylamine |
Rat |
Liver, nasal cavity, |
Pickled/fermented vegetables |
<5.0 |
|
|||||||
(NEMA) |
|
(oesophagus) |
|
(China) |
|
|
|
|
|
|
|
|
|
Me |
Hamster |
Liver, nasal cavity, trachea |
|
|
|
|
|
|
|
|
|
ON |
N |
|
|
|
|
|
|
|
|
|
|
|
|
Et |
|
|
|
|
|
|
|
|
|
|
|
N-Nitrosodiethylamine |
Rat |
Liver, kidney, |
Cured meats (packed |
<2.4 |
|
|||||||
(NDEA) |
|
(oesophagus) |
|
in rubber nettings) |
|
|
|
|
|
|
|
|
|
Et |
Mouse |
Liver, lung, oesophagus, |
Salami |
|
|
|
|
|
|
|
|
ON |
N |
|
forestomach |
|
|
|
|
|
|
|
|
|
Hamster |
Trachea, lung, nasal cavity, |
|
|
<3.9 |
0.6 |
|||||||
|
Et |
|
|
|||||||||
|
|
(oesophagus, forestomach, |
Millet flour and grain |
0.1 |
|
0.5 |
0.22 |
|||||
|
|
|
|
|||||||||
|
|
|
liver |
|
products (China) |
|
|
|
|
|
|
|
1190

|
Rabbit, cat |
Liver, oesophagus |
|
|
|
|
|
|
|
|
Guinea pig, |
Liver |
Dried cuttlefish |
<4.5 |
1.4 |
||||
|
dog, monkey |
|
|
|
|
|
|
|
|
N-Nitrosodibutylamine |
Rat |
Liver, urinary bladder, |
Cured meats (packed |
1 |
|
56 |
10.2 |
||
|
|||||||||
(NDBA) |
|
(oesophagus, pharynx) |
in rubber nettings) |
|
|
|
|
|
|
Bu |
Mouse |
Forestomach, liver, |
Smoked chicken |
<5.3 |
|
||||
ON N |
|
oesophagus, |
Dried fish (Japan) |
|
|
|
|
|
|
Bu |
|
urinary bladder, (lung) |
|
<3.1 |
|
||||
Hamster |
Respiratory tract, lung, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
urinary bladder |
|
|
|
|
|
|
|
|
Guinea pig |
Liver, urinary bladder |
|
|
|
|
|
|
|
N-Nitrosopyrrolidine |
Rat |
Liver, nasal cavity, |
Cured meats |
1.0 |
|
5.0 |
1.8 |
||
|
|||||||||
(NPYR) |
|
(vagina, testis) |
Fried bacon |
<130 |
17 |
||||
|
Mouse |
Lung |
Pickled vegetables |
<96 |
|
|
|||
|
|
|
Mixed spices |
<10 |
|
|
|||
N |
|
|
Dried chillies |
<6.0 |
3.1 |
||||
|
|
Broiled squid |
2.4 |
|
13 |
|
|||
|
|
|
|
||||||
NO |
|
|
|
|
|
|
|
|
|
N-Nitroso-3-hydroxy- |
Rat |
Liver |
Cured meats |
<7.0 |
|
||||
pyrrolidine (NHPYR) |
|
|
Fried bacon |
0.4 |
|
3.9 |
2.2 |
||
|
|
|
|||||||
OH |
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
NO |
|
|
|
|
|
|
|
|
|
N-Nitrosopiperidine |
Rat |
Liver, oesophagus, |
Cured meats |
<20 |
|
|
|||
(NPIP) |
|
upper respiratory |
Fried bacon |
<9.2 |
|
||||
|
|
and digestive tracts, |
|
|
|
|
|
|
|
|
|
nasal cavity |
Peppered salami |
<30 |
|
5.8 |
|||
N |
Mouse |
Forestomach, liver, lung |
Pepper |
<300 |
|
||||
Hamster |
Liver, upper respiratory |
Mixed spices |
0.6 |
|
3.5 |
|
|||
|
|
||||||||
NO |
|
and digestive tracts |
Pickled vegetables |
<14 |
|
|
|||
|
|
|
|
|
|
|
|
|
|
(continued overleaf )
1191

TABLE 7. (continued)
|
|
|
Carcinogenicity |
|
|
|
|
|
|
|
|
|
(following oral administration) |
|
Major dietary source (mg/kg) |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Concentration |
|
|
|
|
|
|
|
|
|
|
|
|
N-Nitroso compound |
Species |
Organotropy |
|
Foodstuff |
|
Range |
Mean |
|||
|
|
|
|
|
|
|
|
|
||
N-Nitrosothiazolidine |
Rat |
Non-carcinogenic |
|
Cured meats |
<32 |
|
|
|||
(NTHZ) |
|
|
|
Fried bacon |
<30 |
|
8.9 |
|||
S |
|
|
|
Smoked fish |
<6 |
|
|
|
||
|
|
|
|
Smoked oyster |
<109 |
|
||||
N |
|
|
|
|
|
|
|
|
|
|
NO |
|
|
|
|
|
|
|
|
|
|
N-Nitroso-2-hydroxymethyl- |
|
No data available |
Cured meats |
Occasionally |
|
|||||
thiazolidine (NHMTHZ) |
|
|
Smoked ham |
<2.8 |
|
|||||
|
S |
|
|
|
|
|
|
|
|
|
HOCH2 |
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NO |
|
|
|
|
|
|
|
|
|
N-Nitrosomorpholine |
Rat |
Liver, (kidney, ovary) |
Packaging contamination of: |
|
|
|
|
|
||
(NMOR) |
|
Mouse |
Liver, lung |
|
Fats/margarine |
1.7 |
|
3.8 |
|
|
|
|
|
|
|||||||
O |
|
Hamster |
Liver, upper respiratory |
|
Dairy products |
<3.2 |
|
|||
|
|
|
tract, colon |
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
NO |
|
|
|
|
|
|
|
|
|
|
N-Nitrososarcosine (NSAR) |
Rat |
Oesophagus |
Cured meats |
<410 |
|
|||||
|
Me |
Mouse |
Lung, nasal cavity, |
Pickled vegetables |
<36 |
|
|
|||
ON N |
|
|
(small intestine) |
Brewing malt |
Occasionally |
|
||||
|
|
|
|
|
|
|
|
|
|
|
CH2 COOH
1192

N-Nitrosoproline (NPRO) |
Rat |
Non-carcinogenic |
Cured meats |
20 |
|
|
580 |
140 |
|
|
|||||||||
|
Mouse |
Non-carcinogenic |
Preserved fish |
<89 |
|
||||
COOH |
|
|
Broiled squid |
<94 |
|
||||
|
|
Dried vegetables |
<24 |
|
|||||
N |
|
|
|
||||||
|
|
|
Dried chillies |
<132 |
|
||||
NO |
|
|
Brewing malt |
<113 |
|
||||
N-Nitroso-4-hydroxy- |
|
|
Beer |
1 |
|
6 |
|
1.7 |
|
|
|
|
|
||||||
Rat |
Non-carcinogenic |
Cured meats |
10 |
|
|
560 |
|
||
|
|
|
|||||||
proline (NHPRO) |
Mouse |
Non-carcinogenic |
|
|
|
|
|
|
|
HO
|
N |
COOH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NO |
|
|
|
|
|
|
|
N-Nitrosothiazolidine- |
No data available |
Cured meats |
<1620 |
|
||||
4-carboxylic acid (NTCA) |
|
Fried bacon |
<14,000 |
85 |
||||
S |
|
|
|
Smoked poultry |
<1240 |
|
||
|
|
|
|
Smoked fish |
<1600 |
67 |
||
N |
COOH |
|
Smoked oyster |
<167 |
|
|||
|
Smoked cheese |
5 |
|
24 |
|
|||
|
|
|
|
|
||||
NO |
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
N-Nitroso-2-methylthia- |
No data available |
Cured meats |
<28 |
|
||||
zolidine-4-carboxylic acid |
|
Smoked poultry |
<98 |
|
||||
(NMTCA) |
|
|
|
|
|
|
|
|
|
S |
|
|
|
|
|
|
|
Me |
|
COOH |
|
|
|
|
|
|
N
NO
(continued overleaf )
1193
1194
TABLE 7. |
(continued) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carcinogenicity |
|
|
|
|
|
|
|
|
|
|
(following oral administration) |
|
Major dietary source (mg/kg) |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Concentration |
|
|
|
|
|
|
|
|
|
|
|
||
N-Nitroso compound |
Species |
Organotropy |
|
Foodstuff |
|
|
Range |
Mean |
|||
|
|
|
|
|
|
|
|||||
N-Nitroso-2-hydroxymethyl- |
|
No data available |
Cured meats |
<2100 |
|
||||||
thiazolidine-4-carboxylic |
|
|
Smoked cheese |
<1628 |
|
||||||
acid (NHMTCA) |
|
|
Smoked poultry |
<462 |
|
||||||
|
|
S |
|
|
|
|
|
|
|
|
|
HOCH2 |
|
COOH |
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
NO |
|
|
|
|
|
|
|
|
|
N-Nitrosooxazolidine-4- |
|
No data available |
Cured meats |
40 |
|
70 |
|
||||
|
|
|
|||||||||
carboxylic acid (NOCA) |
|
|
|
|
|
|
|
|
|
||
O |
|
|
|
|
|
|
|
|
|
|
|
N |
COOH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NO |
|
|
|
|
|
|
|
|
|
|
|
N-Nitroso-5-methyloxazo- |
|
No data available |
Cured meats |
30 |
|
120 |
|
||||
|
|
|
|||||||||
lidine-4-carboxylic acid |
|
|
|
|
|
|
|
|
|
||
(NMOCA) |
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
Me |
N |
COOH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NO

N-Nitroso-N- |
Mice |
Forestomach |
Moldy millet and |
<1.2 |
|
||
(1-methylacetonyl)- |
|
(tested by feeding with |
wheat flour |
|
|
|
|
2-methylpropylamine |
|
amine C nitrite) |
|
|
|
|
|
(NMAMPA) |
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
NO |
|
|
|
|
|
|
|
N-Nitroso-N-(1-methyl- |
|
No data available |
Millet flour and |
0.1 |
|
1.3 |
0.2 |
|
|
||||||
acetonyl)-3-methylbutyl- |
|
|
grain products |
|
|
|
|
amine (NMAMBA) |
|
|
(China) |
|
|
|
|
O
N
NO
Reproduced with permission from Reference 83.
1195
1196 |
H. K. Chagger and A. Williams |
|
|
Bacteria |
|
Macrophages |
Endothelial cells |
(30-90% strains from |
(activated by LPS, BCG) |
(bradykinin) |
|
human sources) |
|
|
|
NO3 , NO2 |
Arginine |
Arginine |
Nitric oxide
Nitrosating agent
NOC
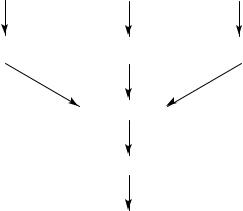
FIGURE 14. Proposed scheme of formation of NOC by bacterial activated macrophages and endothelial cells through intermediate nitric oxide
nitrates or nitrites, probably through formation of nitric oxide at natural pH. Activated macrophages use arginine as a source to produce nitric oxide. Once generated, nitric oxide can be oxidized to nitrosating agents that form NOC readily in the presence of amines. A similar reaction is thought to occur also in endothelial cells.
V. AMINES
Several amino compounds are being used extensively in industrial processes. Most of these compounds are manufactured, except hydrazine. Azo dyes are produced by diazotization of aromatic amines and currently there are at least 3000 azo dyes in use. These dyes are used widely in textiles, leather, printing, paper making, drug and food industries. In the past three decades many food, drug and cosmetic colours have been banned from commercial use as food colourants. This section gives a brief account of adverse affects caused by the use of various amino compounds.
A. Hydrazine
The only known natural source of hydrazine is found in tobacco plants and it may also occur as an intermediate in biological nitrogen fixation. Alkaline solutions of hydrazine in water can be subject to autooxidation by dissolved oxygen, leading to increased hardness and high pH. Decomposition of hydrazine and the increase of pH just below 7 have adverse effects on the marine life ecology. Frog spawn showed teratogenic effects, the embryos of fathead minnows showed deformities and the rainbow trout had poor fitting jaws, pronounced mouth gap and absence of body movement. In air, hydrazine can be oxidized by ozone and hydroxyl radicals. Hydrazine can also be co-metabolised to nitrogen gas by the nitrifying bacterium Nitrosomonas. Germination of seeds of brush squash, peanut and
25. Environmental aspects of compounds |
1197 |
corn was inhibited upon exposure to hydrazine and led to wilting and subsequently death of the plant2.
NOC might be generated by aerial oxidation of the hydrazines. Experimental studies on oxidation of 1,1-dimethylhydrazine which is not a mutagen and N-aminopiperidine which is a mutagen indicated that the oxides of both hydrazide samples were mutagenic103.
B. Azo Dyes
These dyes are used extensively and can be divided into four different categories: azo dyes containing a nitro group, azo dyes containing benzeneamines and related chemicals, azo dyes containing a benzidine group and miscellaneous azo dyes. This section will not lay too much emphasis on the subject of azo dyes as several reviews exist on this topic104,105. Some of the dyes are not mutagenic but become mutagenic through intestinal microflora mechanism for mammalian azo reduction and chemical reduction. It is also possible that the carcinogenesis occurs due to formation of aromatic amines, formed by enzymatic cleavage of azo bonds with subsequent N-ring hydroxylation and N-acetylation of aromatic amines. Table 8 gives a list of dyes which were shown to be carcinogenic and mutagenic to animals and humans106. The C sign indicates that metabolic activation was required and the sign shows cases where no activation was required, i.e. which are direct mutagens.
VI. CONTROL AND LEGISLATION
Most of the pollution of nitroso and other nitrogen related compounds occurs mainly through anthropogenic sources, the largest contributor being combustion. Hence, legislation and control is basically oriented around controlling the amount of dry NOx input into the atmosphere. The estimated global NOx arising from anthropogenic sources is of the order 100 ð 106 t/a, and it is increasing every year. By the year 2020 the expected NOx level is calculated to go up by a factor of 15%. In developed countries like Canada, USA, Europe and Japan limits and guidelines have been specified for the NO/NO2 concentration levels. Amongst the developing countries China is one of the biggest NOx contributors; the total emission is around 6.77 Tg nitrogen per year107. The NOx level is in the developing countries are quite high due to lack of legislative and strategic policies. In order to control the increasing levels of NOx these countries need to implement specified levels of NOx concentrations that should be emitted into the atmosphere. To date, several reviews exist on the control technologies of NOx 6, the issue is to reduce the formation of nitroso and nitro compounds which are formed inadvertently in industrial processes or in the atmosphere. In case of the rubber industry, new accelerators are being tested which are relatively safe as they do not form carcinogenic nitrosamines108. Some of the straight-chain accelerators do not produce detectable amounts of environmentally undesirable N-nitrosoamines. The main source of NDMA and other nitrosamines occurs in food and beverages when direct fire is employed. This has been controlled by reducing the nitrate concentration in the foods and by employing ultra-low NOx burners. The formation of nitroso compounds in cosmetic products can be reduced by elimination of secondary amines as cosmetic ingradients, reduction of the levels of secondary amines and nitrite in the raw products, and avoiding contamination of cosmetics and raw materials with oxides of nitrogen. The federal government in the USA is imposing and amending regulations to reduce the discharge of nitroso compounds into the atmosphere. Besides this, smoking contributes to a high level of indoor pollution, hence most offices and work places are being designated as non-smoking zones in order to cut down the nitroso levels.

1198
TABLE 8. Mutagenic azo dyes: chemical structures and requirements for metabolic activation106
Common |
|
|
Chemical |
|
|
name |
|
|
structure |
|
|
Azo Dyes Containing Nitro Groups |
|
|
|
|
|
|
|
|
N2 O |
|
|
Alizarin Yellow G G |
|
O2 N |
|
O |
|
|
|
|
|
|
|
|
|
|
N N |
OH |
|
|
|
|
HO |
|
|
Acid Alizarin Yellow R |
|
|
|
O |
|
|
|
|
|
|
|
|
|
O2 N |
N N |
OH |
|
Red GTL |
O2 N |
N N |
N |
C2 H2 |
|
+ |
− |
||||
(C.J. Basic Red 18) |
|
|
|
C2 H4 H (CH3 )3 X |
|
|
|
OH |
OH |
Cl |
|
Orasol Navy Blue 2RB |
|
|
N N |
|
CO |
|
|
|
|
||
O2 N
Cl
2
Metabolic activationa
š
š
Azo Dyes Containing Benzeneamines and Related Compounds
NH
Chrysoidin |
|
|
C |
(C.I. Index |
11270) |
N N |
NH2 |
