
Molecular Sieves - Science and Technology - Vol. 6 - Characterization II / 03-Characterization of the Pore Size of Molecular Sieves Using Molecular Probes
.pdfCharacterization of the Pore Size of Molecular Sieves |
123 |
||
Table 3 Adsorption properties of zeolite MFI [16, 19, 51–63] |
|
||
|
|
|
|
Compound |
Adsorption in MFI zeolites |
Refs. |
|
|
|
|
|
n-Hexane |
Yes |
[19, 53, 54, 57, 59, 62] |
|
3-Methylpentane |
Yes |
[19, 53, 54, 62] |
|
Benzene |
Yes |
[16, 19, 51, 53, 57, 58] |
|
Toluene |
Yes |
[16, 54, 58] |
|
p-Xylene |
Yes |
[16, 19, 51, 52, 54, 56–61] |
|
Cyclohexane |
Yes |
[19, 53] |
|
Ethylbenzene |
Yes |
[55, 57] |
|
1-Methylbutylbenzene |
Yes |
[51] |
|
Methylnonanes |
Yes |
[52] |
|
1,4-Diisopropylbenzene |
Yes |
[56] |
|
2,3-Dimethylbutane |
Yes |
[54] |
|
1,2,4-Trimethylbenzene |
Yes |
[19, 59] |
|
Cresol isomers |
Yes |
[56] |
|
2-Methylnaphthalene |
Yes |
[63] |
|
m-Xylene |
Yes 1 |
[52, 53, 55–57, 59] |
|
Neopentane |
Yes 2/no 3 |
[51, 52] |
|
2,2-Dimethylbutane |
Yes 4/no 5 |
[19, 53, 62] |
|
o-Xylene |
Yes 2,4/no 3,6 |
[19, 51, 52, 57, 59, 60] |
|
1,3-Diisopropylbenzene |
No |
[56] |
|
1-Methylnaphthalene |
No |
[63] |
|
cis-Decalin |
No |
[51] |
|
trans-Decalin |
No |
[51] |
|
Tetramethylsilane |
No |
[51] |
|
Cyclooctane |
No |
[51] |
|
1-Ethylpropylbenzene |
No |
[51] |
|
Mesitylene |
No |
[16, 19, 51, 54] |
|
|
|
||
1 |
Not adsorbed during competitive adsorption of all xylene isomers at 283 K in the liquid |
||
phase [56] |
|
|
|
2 |
Adsorption at room temperature in the liquid phase [51] |
|
|
3 |
Adsorption at 333 K in the gas phase [52] |
|
|
4 |
Adsorption at 373 K in the gas phase [19] |
|
|
5 |
Adsorption at room temperature [53] or at 323 K [62] in the gas phase |
||
6 |
Adsorption at 303 K in the liquid phase [60] |
|
|
p-xylene. Namba et al. [56] performed competitive adsorption experiments of xylene isomers in a batch adsorber in the liquid phase using as solvent 1,3,5- triisopropylbenzene, the molecular dimension of which was too large to enter the pores of the zeolite. This technique may eliminate the effect of the external surface, because the solvent, the concentration of which is much higher
124 |
Y. Traa et al. |
than that of the adsorptives, covers the external zeolite surface. At 283 K, only p-xylene was adsorbed on zeolite H-ZSM-5, whereas at higher temperatures all three isomers were adsorbed, even though the p-xylene adsorption capacities were much higher than those of o- and m-xylene. Dessau [51] obtained similar results with xylenes at the same experimental conditions. During competitive adsorption of n-alkanes and aromatics on ZSM-5, he observed a marked preference for the n-alkanes, in distinct contrast to adsorption studies on faujasites. In counterdiffusion studies, in which p-xylene was adsorbed initially, it was rapidly displaced upon addition of n-nonane, because this hydrocarbon has a higher molecular weight. The author argued that this behavior of ZSM-5 might be due to the fact that, unlike A and Y zeolites, it contains no large cavities in which n-alkanes can coil around themselves. The coiling of high molecular weight alkanes inside A and Y zeolites should result in an additional entropy loss upon sorption, thereby reversing the normal order of preferential adsorption of the higher molecular weight component.
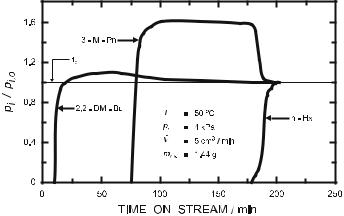
Weitkamp et al. [62, 63] performed competitive adsorption experiments in a flow-type fixed-bed adsorber in the gas phase. During adsorption of an n-hexane/3-methylpentane/2,2-dimethylbutane mixture over Na-ZSM-5, 2,2-dimethylbutane broke through immediately (cf. Fig. 3), i.e., this hexane isomer could not enter the zeolite pores. 3-Methylpentane and n-hexane had access to the pore system, but n-hexane was adsorbed preferentially. Therefore, 3-methylpentane reached partial pressures pi at the adsorber outlet, which were higher than the partial pressure pi,0 at the adsorber inlet, because it was displaced from the zeolite pores by n-hexane [62]. During adsorption of a 2-methylnaphthalene/1-methylnaphthalene mixture on zeolite H-ZSM-5, the bulkier 1-methylnaphthalene was not adsorbed at 100 ◦C, whereas
Fig. 3 Breakthrough curves for the adsorption of an n-hexane/3-methylpentane/2,2- dimethylbutane mixture over zeolite Na-ZSM-5 (n-Hx = n-hexane, 3-M-Pn = 3-methyl- pentane, 2,2-DM-Bu = 2,2-dimethylbutane)
Characterization of the Pore Size of Molecular Sieves |
125 |
2-methylnaphthalene could enter the zeolite pores [63]. On zeolite H-ZSM-12, both isomers were adsorbed, the larger isomer breaking through first. On zeolite Na-Y, the interaction between the zeolite and 1-methylnaphthalene was stronger than that with 2-methylnaphthalene. Therefore, the smaller 2-methylnaphthalene was displaced by 1-methylnaphthalene.
Another medium-pore zeolite that was characterized by adsorption is zeolite MCM-22 (MWW) [20, 24] and its pure silica analogue ITQ-1 (MWW) [64]: Corma et al. [24] determined adsorption isotherms of different probe molecules in the gas phase. The uptake of m-xylene was about half the value for toluene. The value for o-xylene was much lower and approximately equal to that of 1,2,4-trimethylbenzene. These results prompted the authors to suggest the existence of micropores and cavities of two different sizes, i.e., narrower micropores to which only toluene has access and wider micropores or cavities which are penetrated by toluene as well as m-xylene. The much lower uptake of o-xylene and 1,2,4-trimethylbenzene was ascribed to adsorption at the entrances of the micropores and cavities. In another series of experiments, toluene adsorption isotherms were recorded after preadsorption of other adsorptives and an intermittent outgassing. The toluene uptake was lower when preadsorption had been performed, the effect being less important for preadsorbate molecules of larger size [24]. Ravishankar et al. [20] found that the adsorption capacities of n-hexane, cyclohexane, m-xylene and mesitylene of zeolite MCM-22 were virtually independent of the aluminum content. MCM-22 adsorbed moderate amounts of mesitylene, even though this hydrocarbon should not be able to enter the 10-membered-ring pore openings. Therefore, the authors suggested that some of the large 12- membered-ring cavities should be accessible from the external surface, either through defect centers or through the presence of some 12-membered-ring cavities at the external surface [20].
3.3.3
Large-Pore and Extra-Large-Pore Zeolites
For large-pore zeolites, a suitable choice of probe molecules for pore size characterization are tertiary alkylamines with alkyl groups of varying bulkiness or with perfluorinated alkyl groups [65]. Adsorption of the criticallysized molecule perfluorotriethylamine, (C2 F5)3N, showed that the effective aperture of the dehydrated mineral faujasite is substantially smaller than that of synthetic zeolite Y, since its adsorption capacity was much smaller. Perfluorotriethylamine was not adsorbed at all on Ca-X and Ca-Y zeolite. Li-X, Na-X and Cs-X adsorbed (C2F5)2NC3F7 (σ = 0.77 nm), (C3 H7)3N (σ = 0.81 nm) and (C4 H9)3N (σ = 0.81 nm), but not (C4F9)3N (σ = 1.02 nm), whereas Ca-X and Ba-X only adsorbed (C2F5)2NC3F7. From this, the authors concluded that the effective pore diameter of Na-X is about 0.9 to 1.0 nm and that of Ba-X or Ca-X 0.8 to 0.9 nm [65].
126 |
Y. Traa et al. |
Davis et al. [66] distinguished extra-large-pore zeolites from large-pore zeolites by adsorption experiments with 1,3,5-triisopropylbenzene; this molecule was too bulky to enter the pore system of faujasite with reasonable uptake rates, but did have easy access to the channels of VPI-5.
4
Catalytic Test Reactions
Catalytic test reactions for probing the pore width of porous materials have much in common with adsorption tests with the same objective. Adsorption, of course, does occur during these catalytic tests as well, but in addition, a chemical reaction takes place in a shape-selective manner. In order to design and understand test reactions for probing pore dimensions, the fundamentals of shape-selective catalysis should be addressed.
4.1
Shape-Selective Catalysis in Microporous Materials
Various definitions have been used for shape-selective catalysis. Of these, the most straightforward and useful one is as follows [2]: Shape-selective catalysis encompasses all effects in which the selectivity of a catalytic reaction depends, in an unambiguous manner, on the pore width or pore architecture of the porous solid. A comprehensive review of the fundamentals of shape-selective catalysis has been published recently [67]. The shape selectivity effects observed experimentally are usually classified into three types, namely reactant, product and restricted transition state shape selectivity [68].
Both reactant and product shape selectivity have their origins in mass transfer limitations, i.e., in the hindered diffusion of reactant and product molecules, respectively, in the pores of the zeolite catalyst. Therefore, we refer to those two shape selectivity effects as mass transfer shape selectivity. One example of the reactant type of mass transfer shape selectivity, the competitive cracking of n-octane and 2,2,4-trimethylpentane, is depicted in Fig. 4. This situation can be understood as molecular sieving combined with catalytic conversion: 2,2,4-trimethylpentane is too bulky to enter the pores of the zeolite and is, therefore, hindered from reaching the catalytically active sites inside the pores of the zeolite. This molecule can only be converted at catalytic sites located on the external surface of the zeolite crystallites, or it can leave the reactor without being converted. By contrast, the slender n-octane does have access to the pores of the zeolite, where it is readily converted. The net effect, which can be detected at the reactor outlet, is the selective cracking of n-octane into, e.g., an n-butene and n-butane, which are slender molecules that do not experience diffusional hindrance on their way out of the zeolite pores.

Characterization of the Pore Size of Molecular Sieves |
127 |
Fig. 4 Selective cracking of n-octane in the presence of 2,2,4-trimethylpentane as an example of reactant shape selectivity
As an example of the product type of mass transfer shape selectivity, the acid-catalyzed ethylation of toluene has been chosen, and is shown in Fig. 5. Product shape selectivity is the reverse of reactant shape selectivity: Both reactants are small enough to enter the zeolite pores, but of the potential products (o-, m- and p-ethyltoluene), only the slim p-ethyltoluene is small enough to leave the pore system. The two bulkier ethyltoluene isomers, even though they may form in relatively spacious intracrystalline cavities or at channel intersections, are unable to escape from the pores and do not occur in the reactor effluent. Ultimately, these entrapped product molecules may be catalytically transformed into more slender ones (e.g., isomerized into p-ethyltoluene), which are able to leave the pores, or into coke, which is deposited inside the pores. Webster et al. [9] distinguished two types of product shape selectivity: Preferential-diffusion product shape selectivity occurs, if two or more reaction
Fig. 5 Selective formation of p-ethyltoluene in the alkylation of toluene with ethylene as an example of product shape selectivity
128 |
Y. Traa et al. |
products formed within the confines of the structure have effective diffusivities that sufficiently differ from each other, thus allowing one product to preferentially diffuse out of the structure. Often, the lower diffusivity of the bulkier isomer allows for isomerization to the diffusion-preferred (smaller) product. Size-exclusion product shape selectivity arises from an actual confinement of a reaction product within the structure of the solid.
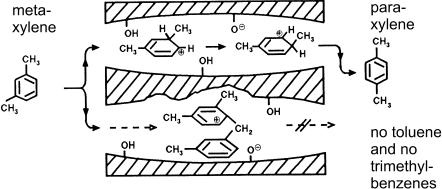
A typical example of restricted transition state shape selectivity is depicted in Fig. 6. m-Xylene can undergo acid-catalyzed isomerization into p-xylene (and o-xylene, which is omitted in Fig. 6 for clarity) and transalkylation into toluene and one of the trimethylbenzene isomers. It is evident that transalkylation is a bimolecular reaction and as such it necessarily proceeds via bulkier transition states and intermediates than the monomolecular isomerization. In a zeolite with the appropriate pore width, there will be just enough space for the accommodation of the transition states and intermediates for the monomolecular reaction, but no room for the formation of the bulky transition states and intermediates of the bimolecular reaction, the net effect being a complete suppression of the latter reaction. As opposed to mass transfer shape selectivity, restricted transition state shape selectivity is due to intrinsic chemical effects, which emerge from the limited space around the intracrystalline active sites.
As long as one assumes that there is no catalytic contribution from the external surface, the influence of the crystallite size is as follows: If mass transfer shape selectivity is operative, the length of the intracrystalline diffusion path and hence the measurable selectivity effects, will decrease with decreasing crystallite size. If restricted transition state shape selectivity is operative, the measurable selectivity will be independent of the crystallite size. If, on the other hand, there is a significant contribution from the non-selective external surface, the influence of the crystallite size on the measurable catalytic
Fig. 6 Suppression of trimethylbenzene plus toluene formation during m-xylene isomerization as an example of restricted transition state shape selectivity

Characterization of the Pore Size of Molecular Sieves |
129 |
selectivity will be more complex. The selectivity will decrease with decreasing crystallite size irrespective of whether the reaction is controlled by mass transfer or restricted transition state shape selectivity. There will only be a gradual difference, which will be difficult to assess.
Several catalytic test reactions for the characterization of the effective pore widths of zeolites have been proposed so far; the results of the best known test reactions were expressed in terms of the quantitative criterions Constraint Index (CI), Refined or Modified Constraint Index (CI ) and Spaciousness Index (SI). Extended review articles on these indices and other test reactions have previously been published [2, 69–71].
4.2
Test Reactions for Monofunctional Acidic Molecular Sieves
4.2.1
Competitive Cracking of n-Hexane and 3-Methylpentane – The Constraint Index, CI
The method of characterizing the effective pore width of zeolites by catalytic tests was first employed by researchers at Mobil Oil Corp. They introduced the Constraint Index [72], which is based on the competitive cracking of an equimolar mixture of n-hexane and 3-methylpentane on the monofunctional acidic form of the zeolite. As long as the catalyst pores are sufficiently spacious, branched alkanes are cracked at higher rates than their unbranched isomers. The opposite holds for medium-pore zeolites, such as H-ZSM-5. Based on this shape selectivity effect, the Constraint Index was defined at Mobil as the ratio of first order rate constants (k) of the cracking of n-hexane and 3-methylpentane:
kn-Hx |
|
CI ≡ k3-M-Pn . |
(1) |
The Constraint Index has been routinely used in Mobil’s patents for about two decades. In the scientific literature, precise experimental conditions for its determination were given (reaction temperature between 290 and 510 ◦C, liquid hourly space velocity (LHSV) between 0.1 and 1 h–1, 10 vol.- % of each reactant in helium as carrier gas, mass of catalyst around 1 g, overall conversion 10 to 60%, fixed-bed reactor at atmospheric pressure) [72]. The reactor effluent is to be analyzed after 20 min time on stream. With the performance equation for integral fixed-bed reactors [73], Eq. 1 can be rewritten as
log(1 – Xn-Hx) |
|
CI ≡ log(1 – X3-M-Pn) |
(2) |
(X = conversion) .
According to Frilette et al. [72], the Constraint Index allows for a classification of zeolites into large-pore (12-membered-ring), medium-pore (10-

130 |
Y. Traa et al. |
membered-ring) and small-pore (8-membered-ring) molecular sieves:
CI < 1 : large-pore materials;
1 ≤ CI ≤ 12 : medium-pore materials;
12 < CI : small-pore materials.
Constraint Indices taken from the literature are summarized in Table 4. By and large, a correct classification of 8-, 10and 12-membered-ring zeolites can be achieved. Exceptions are zeolites ZSM-12 (MTW) and MCM-22 (MWW). ZSM-12 possesses 12-membered-ring pores which are, however, strongly puckered and hence, narrowed, and on the basis of its Constraint Index it would be classified as a material with 10-membered-ring pores. Zeolite MCM-22 possesses large intracrystalline cavities, which are only accessible via 10-membered-ring windows. In this case, the Constraint Index of 1.5 can be looked upon as an averaged value characterizing the mean space available in the 10-membered-ring pores and in the large cavities.
Values for the Constraint Indices of more recent zeolite structures are given in [78], revealing even more shortcomings of the Constraint Index test. One example is that zeolites with 14-membered rings and pore openings greater than 0.8 nm cannot be distinguished from large-pore zeolites, simply because the degree of absence of spatial constraints cannot vary. Another example is that some zeolites with large internal cavities and pores composed of 8- or 9-membered rings do not generate Constraint Indices higher than zeolites with 10-membered-ring pores, probably again because the Constraint Index test averages the space in the pores and the cavities.
Table 4 Constraint Indices for selected zeolites. Data from the patent literature [74, 75], values in parentheses from the open literature [76, 77]. The dashed line is to indicate that zeolite ZSM-12 belongs to medium-pore zeolites if classified after CI even though it is defined as a large-pore zeolite by its ring size
Characterization of the Pore Size of Molecular Sieves |
131 |
Other advantages and disadvantages of the Constraint Index have been discussed in detail in [2]. The reaction can suffer from a relatively fast catalyst deactivation, if large-pore zeolites are used, which makes a reliable determination of the CI difficult, and a (sometimes pronounced) temperature dependence for medium-pore zeolites. The reason for the latter has been investigated in much detail by Haag et al. [79, 80]. These authors showed, in a convincing manner, that two basically different cracking mechanisms can be operative, namely the classical bimolecular chain-type mechanism involving tri-coordinated alkylcarbenium ions and a monomolecular mechanism via non-classical penta-coordinated alkylcarbonium ions. The latter has a higher activation energy, hence its contribution increases with increasing reaction temperature, and due to its requiring less space, the Constraint Index for a given zeolitic material decreases with increasing temperature. However, Macedonia and Maginn [81] recently used Monte Carlo integration methods employing a classical molecular mechanics force field to predict values for the Constraint Indices of 12 different zeolites. These authors concluded that it is not necessary to invoke such a change in mechanism to explain decreasing Constraint Indices with increasing temperature. Their calculations indicated that the impact of confinement on the bimolecular transition state decreases with increasing temperature, effecting a decrease of the Constraint Index. This was said to be caused by a competition between energetic confinement effects that dominate at lower temperatures and entropic effects that become dominant at high temperature.
As to the nature of the shape selectivity effects, Haag et al. [82] demonstrated in a most impressive study with H-ZSM-5 samples of equal concentration of acidic sites but different crystal sizes (0.05 to 2.7 µm) that neither the measurable rate of cracking of n-hexane nor that of the bulkier 3-methylpentane depend on the length of the intracrystalline diffusion paths. From this finding, the selectivity effects encountered in the competitive cracking of n-hexane and 3-methylpentane have to be interpreted in terms of intrinsic chemical effects (i.e., restricted transition state shape selectivity) rather than by mass transport effects. Haag et al. [82] suggested that the rate-controlling step in the chain-type mechanism of acid-catalyzed alkane cracking via carbocations is the chain-propagating hydride transfer between a cracked alkylcarbenium ion and a feed molecule, requiring significantly more space for the transition state if the feed alkane is branched, the net effect being a significant inhibition of cracking of 3-methylpentane. The simulations performed by Macedonia and Maginn [81] indicated, however, that for zeolites, the pores of which are too small to accommodate the bimolecular transition state, such as ZSM-23 (MTT) and ferrierite (FER), the monomolecular mechanism dominates, with the measured Constraint Index attributed to reactant shape selectivity. Only for zeolites, the pores of which are large enough for the bimolecular transition state but small enough for confinement effects, the bimolecular reaction was predominant, and the selectivity was based on restricted transition state shape selectivity.

132 |
Y. Traa et al. |
Recently, Baeck et al. [32] demonstrated that the Constraint Index is useful for probing subtle changes of the pore size as effected by CVD. The Constraint Index determined on zeolite Mg-ZSM-22 (TON) increased from about 9.9 to about 13.3 after 3 h of deposition of tetraethyl orthosilicate, reflecting the decrease of the pore opening by CVD.
In conclusion, with the Constraint Index, the idea of probing the pore width by catalytic test reactions was introduced into zeolite science. It no doubt fostered the search for alternative and improved catalytic test reactions. In spite of its shortcomings enumerated above, the Constraint Index test has been widely used, and ample data is available in the literature.
4.2.2
Isomerization and Disproportionation of m-Xylene
On acidic catalysts, m-xylene can undergo isomerization into o- and p-xylene and disproportionation (or transalkylation) into toluene and 1,2,3-, 1,2,4- or 1,3,5-trimethylbenzene (cf. Fig. 7). While it is obvious that disproportionation is necessarily a reaction involving a bimolecular transition state, there is some ambiguity as to whether the acid-catalyzed isomerization of xylenes proceeds via a monomolecular or a bimolecular pathway [83, 84].
The use of m-xylene conversion for the characterization of the effective pore width of zeolites was first proposed by Gnep et al. [85]. These authors identified three selectivity criteria that may furnish valuable information on the effective pore width: (i) the relative rates of formation of o- and p-xylene, (ii) the ratio of rates of disproportionation and isomerization and (iii) the distribution of the trimethylbenzene isomers formed in the disproportionation reaction.
Criterion (i) is based on the finding that, in the absence of shape selectivity, o- and p-xylene are formed at virtually the same rate. With decreasing pore width, however, the formation of p-xylene is increasingly favored over the formation of the bulkier o-xylene [85]. This effect is best interpreted in terms of product shape selectivity, i.e., progressively hindered diffusion of o-xylene molecules, as the pores become narrower.
Fig. 7 Principal reaction pathways of m-xylene over acidic catalysts
