
- •Федеральное агентство по образованию
- •Государственное образовательное учреждение высшего профессионального образования
- •Санкт-Петербургский государственный горный институт им. Г.В. Плеханова
- •(Технический университет)
- •Английский язык
- •Санкт-Петербург
- •Text 2 Oil and Gas Composition
- •Petroleum hydrocarbon structures
- •1. Paraffins
- •2. Naphthenes
- •3. Aromatics
- •Text 3 Oil and Natural Gas Formation
- •Text 4 Characteristics of Reservoir Rock
- •Text 6 Finding the oil
- •Oil and Gas Quiz
- •Unit 2.
- •From the history of oil industry
- •Petroleum History
- •Petroleum Timeline
- •Ex.4 After reading the following texts 3-7 make the similar Petroleum Timeline for our country. Text 3 From the History of Oil Production in Russia
- •Text 4 The Birth of the Industry
- •Text 5 The Rise of the Soviet Oil Industry
- •Text 7 Further Development
- •Text 8 Modern Development of Oil and Gas Industry in Russia
- •Text 9 The Role of Oil and Gas in Our Life
- •Text 10 Oil and Gas Consumption
- •Text 11 The Future of Oil
- •Text 12 Oil Prices
- •Text 2 How to Get a Job in the Oil Industry
- •Text 3 The Petroleum Industry
- •Oil Industry has Lost its Luster?
- •Contents
- •Bibliography:
Text 2 Oil and Gas Composition
hydrocarbons – углеводороды
to devour, v – поглощать, уничтожать
aerobic bacteria – анаэробные бактерии
paraffins – парафиновые углеводороды
branched carbon rings – разветвленные кольца углерода
to saturate, v – насыщать
stable compound – стойкое соединение
naphthenes – нафтеновые углеводороды
side chain- боковая цепь
aromatics- ароматические углеводороды
to attach, v – присоединять, прикреплять
boiling point –точка кипения
The oil and gas consist of hydrocarbons, molecules composed of carbon and hydrogen. These hydrocarbons cannot exist for very long at the surface of Earth because they are attacked by oxygen and devoured by bacteria that live in surroundings where air is present (aerobic bacteria). Thus, they are quite rapidly transformed into carbon dioxide (CO2) and water. Incidentally, hydrocarbons do not exist in deep layers of the Earth because, beyond a certain depth (around 10 km), they would be destroyed, since the temperature is too high (the further you plunge underground, the hotter it becomes).
Petroleum hydrocarbon structures
Petroleum consists of three main hydrocarbon groups:
1. Paraffins
These consist of straight or branched carbon rings saturated with hydrogen atoms, the simplest of which is methane (CH4) the main ingredient of natural gas. Others in this group include ethane (C2H6), and propane (C3H8).
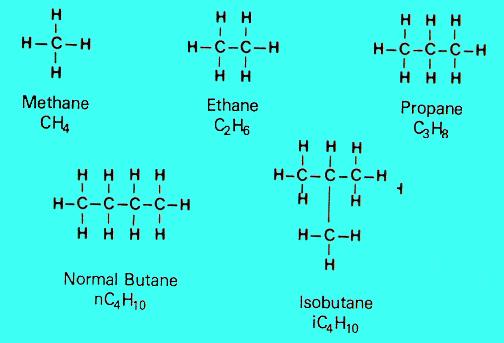
Chemically paraffins are very stable compounds.
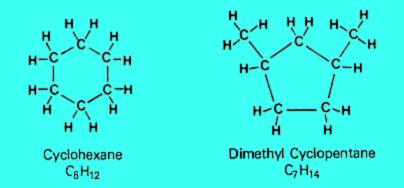
2. Naphthenes
Naphthenes consist of carbon rings, sometimes with side chains, saturated with hydrogen atoms. Naphthenes are chemically stable, they occur naturally in crude oil and have properties similar to paraffins.
3. Aromatics
Aromatic hydrocarbons are compounds that contain a ring of six carbon atoms with alternating double and single bonds and six attached hydrogen atoms. This type of structure is known as a benzene ring. They occur naturally in crude oil, and can also be created by the refining process.
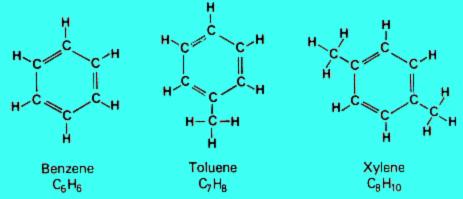
The more carbon atoms a hydrocarbon molecule has, the "heavier" it is (the higher is its molecular weight) and the higher is its boiling point.
Small quantities of a crude oil may be composed of compounds containing oxygen, nitrogen, sulphur and metals. Sulphur content ranges from traces to more than 5 per cent. If a crude oil contains appreciable quantities of sulphur it is called a sour crude (at least 2.5% sulfur); if it contains little or no sulphur it is called a sweet crude (sulfur content less than 0.5%).
Ex.1 Answer the following questions:
Where can hydrocarbons exist?
What are the main hydrocarbon groups?
How does the number of carbon atoms influence hydrocarbon molecular properties?
What is a benzene ring?
How is crude oil classified into sweet and sour?
Text 3 Oil and Natural Gas Formation
Ex.1 Read and translate the text.
Ex.2 Translate the following word combinations and make up your own sentences with them:
organic matter, the surface of the earth, carbon dioxide, poorly oxygenated environment, foul-smelling mud, exceptional conditions, source rock, impermeable rock, profitable exploitation, sufficient quantities, petroleum engineers
Ex.3 Supply the second part of the text with a suitable heading and retell it.
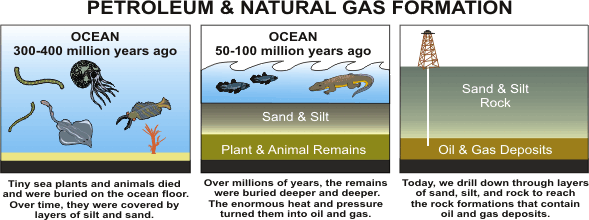
I
So, where do these hydrocarbons come from? Their composition shows that they result from a transformation of organic matter consisting of living organisms that died a long time ago. When a plant or an animal dies on the surface of the earth, other living creatures generally recycle its matter. What is not devoured by predators, like vultures or bacteria, is oxidised into carbon dioxide and water, and this carbon dioxide feeds the growth of new plants. Nevertheless, a tiny part, perhaps 0,1%, of this organic mass escapes from the implacable cycle. In certain cases the remains of dead beings sink to the bottom of the seas. In this environment, very calm and poorly oxygenated, the organic remains are mixed with mineral matter (particles of clay, very fine sand…) to form dark and foul-smelling mud. This bad odour is characteristic of the action of anaerobic bacteria which don’t need air to live.
A part of this organic matter is therefore preserved. The animals that produce it are minuscule or microscopic: principally marine plankton. The plant debris is carried away by the rivers that feed the sea. This organic matter, mixed with mineral sediments, accumulates little by little. For large quantities of oil or gas to be produced later, the proportion of organic matter must be sufficient, that is to say at least 1 to 2%, to constitute the source rock for our oil. 1 to 2 % does not seem a lot, but exceptional conditions are required to attain this percentage: a lot of plankton or plant debris and not too much mineral matter. A warm climate favourable to plankton; the absence of mountains nearby to limit the volumes of mineral sediment; and a delta or mouth of a major river to carry a lot of plant debris…all of these elements contribute to the formation of the source rock. Nevertheless, whilst this rock remains on the surface of the sea floor, it is unable to produce oil.
II
Accumulations of oil and gas – the fields – exist underground almost everywhere in the world. But all the same it is necessary that certain conditions come together for those accumulations to develop. What is called the genesis of oil follows seven fundamental steps, each one essential and above all very, very slow.
1. First, it is necessary to have organic matter capable of being transformed into oil, and in sufficient quantities: this is the source rock.
2. Then all the conditions favourable for the transformation of this potential oil and gas must be present.
3. The new oil and gas then embark on a migration towards the surface.
4. During this migration the hydrocarbons must meet a rock capable of hoarding them in large quantities: the reservoir.
5. This reservoir must be impervious. A barrier (seal or cap) is therefore necessary: that is to say an impermeable rock to prevent the oil and gas from pursuing their way upwards. This rock is the seal or cap rock.
6. Then, to accumulate quantities of oil and gas sufficient for profitable exploitation, the substratum must have a form sufficiently large and with a closed geology: this is the trap.
7. Finally once well tucked away in their trap, our oil and gas must not be destabilised by attacks coming from the exterior. Good conditions for the preservation of hydrocarbons are necessary.
When teams of petroleum engineers study a zone, one of their principal objectives is to determine if these seven steps have, indeed, a good chance of happening. The totality of these seven steps is called an oil system.
