
Sehu - Ophthalmic Pathology-2005
.pdf
230 C H A P T E R 1 0
Retina - Central degeneration / ARMD
Deposits - Basement membrane
degenerate intact photoreceptors
photoreceptors
degenerate RPE basement
membrane deposit
Bruch’s membrane
Picro Mallory V stain |
choriocapillaris |
Picro Mallory V stain |
Figure 10.81
Retina - Central degeneration
ARMD
Deposit - Basement membrane
melanosomes
RPE cell membrane
basal laminar deposit
outer
collagenous basement membrane layer
elastic layer
endothelium |
|
|
|
Normal |
inner collagenous layer |
BMD in ARMD |
|
red cell |
|||
|
|
Figure 10.83
Picro-Mallory V stain
PR layer atrophy
atrophic RPE
remnant of basement membrane deposit
lumen of
venule fibrous component lumen of arteriole
choriocapillaris
Bruch’s membrane
Retina - Central degeneration / ARMD
Wet/Exudative - Subretinal neovascular membrane type 1
Figure 10.85
Retina - Central degeneration / ARMD |
proteinaceous |
Deposits - Basement membrane |
subretinal exudate |
basement membrane deposits
Bruch’s membrane
choriocapillaris
venule
arteriole of choriocapillaris of choriocapillaris
Figure 10.82
Retina - Central degeneration / ARMD
Wet/Exudative - Subretinal neovascular membrane type 1
vessel in |
break in Bruch’s membrane retinal pigment epithelium |
membrane |
vessel in membrane |
basement membrane deposit
feeder vessel from
choroid
Figure 10.84
Figure 10.81 Basement membrane deposit (BMD) is best identified with a special stain (Picro-Mallory V). The deposit is blue and appears striated and the overlying retinal pigment epithelium (RPE) is degenerate. This is associated with degeneration of photoreceptors. On the left, the BMD is thin and the photoreceptors are preserved. On the right, the deposit is thick and the photoreceptors are atrophic.
Figure 10.82 Advanced basement membrane deposits have a striated appearance in light microscopy. In this example, the retinal pigment epithelium cells are swollen beneath a proteinaceous subretinal exudate.
Figure 10.83 By electron microscopy, the “spiky” appearance of the basement membrane deposit (BMD) is apparent between the cell membrane of the retinal pigment epithelium and the basement membrane (right). The left figure is that of the normal appearance in a young individual for comparison.
Figure 10.84 In the early stages of neovascular age-related macular degeneration (ARMD), fibrovascular tissue is located between the retinal pigment epithelium and Bruch’s membrane. In many cases, it is possible to identify basement membrane deposit beneath the retinal pigment epithelium.
Figure 10.85 Organisation of the subretinal pigment epithelial fibrovascular membrane (subretinal neovascular membrane type 1) which contains arterioles and venules is associated with atrophy of the overlying photoreceptor (PR) layer (Picro-Mallory V stain).

R E T I N A 231
A type 2 subretinal neovascular membrane in exudative ARMD refers to an extension of fibrovascular proliferation between the RPE and the neural retina (subretinal space). This potential space provides an easy extension of haemorrhage from the fragile capillaries (Figure 10.86). Continuing leakage and haemorrhage from the capillaries lead to large submacular fibrous scars (Figure 10.87). This is termed a disciform scar. Continuous subretinal traction may lead to retinal distortion and rupture of the retina and its blood vessels may be followed by bleeding into the vitreous.
Haemorrhage may be complicated by a massive retinal detachment (Figure 10.88) which, in the past, has lead to enucleation because the subretinal mass simulated a melanoma (Figure 10.89).
Submacular surgery
It is increasingly frequent for a pathologist to receive specimens from submacular surgery for assumed subretinal neovascularisation. It is a common understanding that the removal of type 2 membranes has a better prognosis than type 1. Histology of excised membranes reveals fibrovascular tissue lined by RPE cells or containing strips of RPE cells (Figure 10.90).
Differential diagnosis of subretinal neovascularisation in ARMD
1Trauma (civil or iatrogenic including overzealous panretinal photocoagulation).
2Presumed ocular histoplasmosis syndrome (POHS).
3High myopia (see below).
4Angioid streaks.
5Any condition that disrupts Bruch’s membrane or the retinal pigment epithelium can potentially lead to subretinal neovascularisation (Figure 10.91).
Epiretinal membrane
Other names: cellophane maculopathy, macular pucker, premacular gliosis.
These are thin contractile membranes derived from intraretinal glial cells which distort the inner limiting membrane of the retina. This condition is often asymptomatic but increasing severity of membrane contracture may lead to decrease or distortion of vision, particularly when the macula is involved (Figure 10.92). A lower threshold for vitreoretinal surgical intervention means that more specimens are submitted to pathology.
Numerous aetiologies have been associated with the formation of an epiretinal membrane:
•idiopathic
•uveitis
•previous trauma including surgery
•diabetes and other vascular diseases.
The composition of the excised membranes will depend on the aetiology but the tissue usually consists primarily of glial cells (Figure 10.92). This entity differs from proliferative vitreoretinopathy (PVR) which is secondary to a previous retinal break with or without rhegmatogenous retinal
detachment. In PVR, the membrane contains pigmented cells derived from RPE (see below).
Myopia
This condition occurs when the plane of focus of the image is in front of the retina. There are two types:
1Index: when the globe dimensions are normal.
2Axial: when it is secondary to elongation of the globe. In the latter variant, elongation of the globe is thought to
be due to factors influencing the stretching of the sclera, but the precise mechanisms are debatable – hereditary, reading, and close work.
Depending on the severity, the clinical features of axial myopia are variable and include: elongation of the globe with possible posterior staphyloma, lacquer cracks, and disc changes (tilting and temporal peripapillary atrophy in the form of a white crescent) due to posterior stretching of the sclera. The pathological features of the optic nerve head are shown in Figures 10.93 and 10.94. Myopic eyes are at a higher risk of rhegmatogenous retinal detachment due to an earlier onset of peripheral retinal degeneration, vitreous syneresis, and detachment (see below).
Peripheral
This section deals with the changes which are seen in the peripheral retina by indirect ophthalmoscopy and in pathology as incidental findings in enucleated globes. These degenerations are usually innocuous but some may progress to retinal detachment.
Lattice
This form of peripheral degeneration has the greatest association with retinal detachment. Small, sharply demarcated linear areas contain an overlying network of white lines (Figure 10.95). Foci of lattice degeneration are patchy in distribution and are most common in the upper temporal quadrant. Histology reveals retinal thinning and hole formation in the region of a hyalinised retinal arteriole (Figure 10.96). There is an absence of vitreous over the defect, although attachments appear to be firm at the edge.
Cobblestone/pavingstone
Indirect ophthalmoscopy and macroscopic pathological examination demonstrate cobblestone degeneration as discrete, yellow, circular areas of chorioretinal atrophy with speckled clumps of hyperpigmentation. These areas expand slowly and may merge with other areas of degeneration (Figure 10.97). At the histological level, the atrophic gliotic retina is fused with Bruch’s membrane and the choroid and choriocapillaris are atrophic. The RPE is absent in the scarred area but is hyperplastic at the periph-
ery where there may be |
intraretinal proliferation |
(Figure 10.97). This form of |
degeneration is found |
most commonly in the inferior peripheral retina and is innocuous.

232 C H A P T E R 1 0
Retina - Central degeneration / ARMD
Wet/Exudative - Subretinal neovascular membrane type 2
GCL gliotic retina overlying type 1 membrane
fibrovascular tissue growing into subretinal space (type 2)
photoreceptor atrophy
haemorrhage
feeder vessel
|
break in RPE |
Figure 10.86 |
|
Retina - Central degeneration |
degenerate |
ARMD |
retina |
|
|
Wet/Exudative - Disciform scar |
|
extensive |
|
retinal detachment |
|
|
fibrovascular tissue |
subretinal haemorrhage |
(disciform scar) |
|
disciform scar
Figure 10.88
fibrovascular tissue
RPE
Retina - Central degeneration / ARMD
Wet/Exudative - Excision of subretinal neovascular membrane
Figure 10.90
Retina - Central degeneration / ARMD |
|
|
Wet/Exudative - Disciform scar |
fibrovascular |
|
|
|
|
degenerate |
scar |
retinal |
|
||
retina |
|
tear with |
|
|
vitreous |
|
|
bleed |
optic |
optic disc |
vitreous |
clumps of |
nerve |
disciform |
bleed |
proliferating RPE cells |
|
|||
|
scar |
|
|
macular branch of posterior ciliary artery
Figure 10.87
subretinal mass
altered
blood cut surface to
show fibrovascular mass
proteinaceous |
obscured |
exudate |
optic disc |
RPE proliferation
Retina - Central degeneration / ARMD
Wet/Exudative - Disciform scar simulating a melanoma
Figure 10.89
Figure 10.86 This type 2 subretinal neovascular membrane (SRNVM) in wet ARMD has arisen from the edge of a type 1 SRNVM in which there is a disruption in the retinal pigment epithelium (RPE). Neovascularisation and haemorrhage have extended between the RPE and a normal outer retina. The presence of a thickened ganglion cell layer (GCL) confirms that the location is the macula area.
Figure 10.87 Contraction of fibrous tissue within this disciform scar leads to macular distortion and tears in the retina from which there is bleeding into the vitreous. The macroscopic appearances are shown in the inset.
Figure 10.88 Bleeding from a relatively small disciform scar leads to extensive haemorrhagic detachment of the retina. The inset shows the histological appearance in this specimen.
Figure 10.89 This archival specimen was an enucleation for a presumed peripapillary choroidal melanoma. Examination of the cut surface (inset) reveals a disciform scar, altered blood, and exudation. The hyperpigmented areas are due to a reactionary proliferation of the retinal pigment epithelium.
Figure 10.90 It is of clinical interest that an excised subretinal neovascular membrane is identified as type 1 or type 2. As can be seen in this specimen, a considerable degree of expertise (and inspired guesswork!) is required to answer this question. However, considering the close relationship between the continuous monolayer of retinal pigment epithelium (RPE) and the fibrovascular tissue, it is likely that this is a type 1 subretinal neovascular membrane (see Figure 10.85 – type 1 SRNVM).

R E T I N A 233
Retina - Central degeneration / ARMD
Wet/Exudative - Neovascular membrane
Extramacular location
neovascular tuft beneath RPE
artefactual retinal detachment
kink in thickened Bruch's membrane
break in calcified Bruch's membrane
Figure 10.91
temporal side |
nasal side |
bare sclera
|
Myopia |
edge of choroid and retina |
Normal |
|
Retina - Myopic degeneration
Peripapillary pathology
Figure 10.93
Retina - Peripheral degeneration
Lattice
pars plana
ora serrata
white lattice network
patchy distribution of lattice
Figure 10.95
Retina - Central degeneration Epiretinal membrane
Macular pucker
epiretinal membrane
distorted macular surface
PAS stain
epiretinal membrane
ILM
INL
GFAP stain
Figure 10.92
gliotic retina
bare sclera
temporal side
nasal side
RPE |
|
Retina - |
proliferation |
nasal ridge |
Myopic degeneration |
Figure 10.94
Figure 10.91 This example of subretinal neovascular membrane type 1 was a chance finding. The Bruch’s membrane is purple in colour and this is assumed by pathologists to represent calcification. When there is kinking, a break in Bruch’s membrane stimulates subretinal pigment epithelial neovascularisation. This appearance is similar to breaks observed in pseudoxanthoma elasticum, Paget’s disease, and angioid streaks.
Figure 10.92 The effects of contraction on the inner retina by a thin glial membrane are shown in this figure (upper, PAS stain). Secondary degenerative changes are present in the inner layers of the macula. The lower figure illustrates glial cell proliferation within an epiretinal membrane and within the inner retina. The immunohistochemical identification of glial cells is by an antibody label for a contractile glial protein (glial fibrillary acidic protein – GFAP) using a brown chromogen. ILM inner limiting membrane, INL inner nuclear layer.
Figure 10.93 An appreciation of the abnormal histological configuration of the optic disc in myopia is better understood when the normal disc is included for comparison (lower). On the temporal side of the myopic disc (upper), the choroid and retina are displaced away from the scleral canal. The temporal peripapillary retina overlies bare sclera and this corresponds to the temporal crescent seen on ophthalmoscopy. On the nasal side, the nerve fibre layer is formed into a prominent ridge. Clinically the myopic abnormality is termed a tilted disc.
Figure 10.94 The inset shows the macroscopic appearance of a myopic disc in which there is considerable temporal atrophy and retinal pigment epithelial (RPE) reactionary proliferation. The main histopathological feature of a myopic disc is the presence of gliotic retina on the temporal side overlying “bare” sclera.
Figure 10.95 Lattice degeneration is easily identified in a pathological specimen as linear oval defects in the peripheral retina. These areas are crossed by fine white lines which represent hyalinised peripheral retinal arcades. The inset shows a detail of an area of lattice degeneration.

234 C H A P T E R 1 0
Retina - Peripheral degeneration
Lattice
Intact atrophic retina |
retinal |
space above |
towards |
|
overlying vitreous |
||||
|
|
|||
within lattice degeneration arteriole |
|
periphery |
||
Edge of hole |
|
Centre of hole |
|
|
RPE |
drusen |
eversion |
|
choroid |
of retina |
||
|
Figure 10.96
Figure 10.96 A composite illustration of different levels taken from the area of lattice degeneration shown in the inset in Figure 10.95. At the edge of the lattice, the retina is thinned and gliotic anterior to a hyalinised blood vessel which is surrounded by retinal pigment epithelium (RPE) cells (upper). The lower left illustration shows a deeper level in the lattice to reveal the changes of detachment and hole formation with photoreceptor atrophy and the effects of vitreous traction on the glial cells on the inner surface of the hyalinised vessel. Lower right, in the
circular areas of
chorioretinal atrophy ora serrata (cobblestones)
hyperpigmentation
RPE |
gliotic retina |
intraretinal |
hyperplasia |
fused with atrophic choroid |
RPE |
|
|
proliferation |
Retina - Peripheral degeneration
Cobblestone
Figure 10.97
centre of the lattice, there is a retinal hole and a defect in the overlying vitreous. Traction on the edge of the retina hole has produced eversion of the gliotic retina.
Figure 10.97 Irregular circular areas of depigmentation occur in the peripheral retina and do not predispose to any complication. Pigmentation is seen at the periphery as well as within the white areas (upper left and right). Histology reveals a gliotic retina fused with an atrophic choroid (lower) – this corresponds with the pale areas seen macroscopically and by indirect ophthalmoscopy.
Peripheral microcystoid
Sheets of microcysts may be present in the retina just posterior to the ora serrata (Figures 10.7, 10.98). The presumed pathogenesis is ischaemia due to degenerative occlusive disease in the peripheral retinal arterioles. The only complication is that the cysts may break down to form a retinoschisis in the outer plexiform layer (Figure 10.99) or, as an extreme rarity, a giant cyst (Figure 10.100).
Retinal breaks (holes/tears/dialysis)
Retinal breaks are full thickness defects in the retina that may result in a rhegmatogenous retinal detachment (see below).
Holes
Holes are full thickness defects of the retina in the absence of vitreous traction. They are usually the result of peripheral atrophic changes (Figure 10.101) and rarely progress to detachment.
Tears
In the ageing eye, the vitreous liquefies (syneresis) and the remaining condensed gel detaches from the posterior retina and disc. Tears are full thickness defects of the retina resulting from persistent traction on focal adhesions between the vitreous and the retinal surface. The break often possesses an overlying flap (operculum) with attached vitreous (Figure 10.102).
Unrelieved traction at the edge of the tear often results in further progression of this condition.
Dialysis
Breaks along the edge between the retina and the ora serrata are called dialyses and are usually large at the time of diagnosis.
Retinal detachment
This is defined as the separation of the neurosensory retina from the underlying RPE.
There are three types of retinal detachment:
1Rhegmatogenous.
2Exudative.
3Tractional – see text on diabetic retinopathy in the section “Diabetes” above.
Rhegmatogenous retinal detachment
Rhegmatogenous (Gk rhegma: a tear) retinal detachment is the result of a full thickness tear in the retina permitting egress of vitreal fluid into the space between the neural retina and the RPE.
Clinical presentation
1 Symptoms: predetachment symptoms include increased visual floaters and/or flashes. With detachment, a partial scotoma is experienced which, if untreated, leads to total loss of vision.
2 Signs: fundus examination shows a mobile detached retina. A break or multiple breaks may also be visible, most commonly in the upper temporal quadrant. The contralateral eye may show peripheral retinal breaks or lattice degeneration in the corresponding quadrant.
If the fundus cannot be visualised, an ultrasound investigation may be necessary.

R E T I N A 235
Retina - Peripheral degeneration
Microcystoid
ora serrata
peripheral microcystoid
autolytic folding of retina
Figure 10.98
blood filled macrocyst arising in a retinoschisis
cornea removed for keratoplasty
detached retina
peripheral
Retina - Peripheral degeneration microcystoid degeneration
Macrocyst
Figure 10.100
towards anterior direction of traction
presistent vitreous attachment
operculum
rounded posterior lip of tear
atrophic photoreceptor layer
Retina - Peripheral break
Small horseshoe tear
Figure 10.102
multiple cysts in OPL
towards anterior
Early
remnants of Müller cells |
Late |
schisis
towards anterior
Retina - Peripheral degeneration Microcystoid
Figure 10.99
Retina - Peripheral break
Hole
meridional
folds
ora serrata
retinal hole
detached edges
peripheral microcystoid degeneration
Figure 10.101
Figure 10.98 In microcystoid degeneration, the cysts adopt a honeycomb pattern particularly when an autopsy globe is fixed in formal saline, which makes the retina opaque.
Figure 10.99 The tiny cysts of microcystoid degeneration form in the outer plexiform layer (OPL) and are located in the peripheral retina. The cysts become smaller in the posterior part of the abnormality (upper). As the cysts enlarge, the inner and outer retinal layers are bridged by surviving Müller cells. When these break down, the two retinal layers split to form a retinoschisis (lower).
Figure 10.100 This archival specimen illustrates a blood-filled retinal macrocyst which was erroneously diagnosed as a malignant melanoma. The practice at that time was to use the cornea for donor purposes.
Figure 10.101 Retinal holes are occasional findings in eyes enucleated for other disorders. In this example, folds of retina project onto the pars plana – socalled meridional folds (upper). Histologically, the edge of a retinal hole is rounded and there is an associated small retinal detachment – note the peripheral microcystoid degeneration (lower).
Figure 10.102 In a rhegmatogenous retinal detachment (see below), it is sometimes possible for a pathologist to identify a retinal tear (inset) with an overlying flap (operculum). In this example, histology through the tear reveals persisting vitreous attachment to the anterior lip of the operculum. The atrophic photoreceptor layer, the rounded edge of the tear, and small cysts within the gliotic anterior lip each indicate longstanding detachment.

236 C H A P T E R 1 0
Aetiology and pathogenesis
Predisposing factors include:
•increasing age
•myopia
•lattice degeneration of the retina
•mechanical trauma (civil and surgical).
In the absence of predisposing factors, the incidence of
retinal detachment is 1:10 000.
The pathogenesis of rhegmatogenous retinal detachment is based on the following factors:
•syneresis with liquefaction of vitreous gel
•persistent vitreous traction
•retinal break
•fluid movement from vitreous into the subretinal space.
Possible modes of treatment
The principles involve surgery to close the retinal break, reattachment of the retina, and relief of vitreous traction. The different procedures are multiple and complex – see the relevant clinical texts.
Surgery is subdivided into the following categories:
1 Conventional: involving explant material to indent the globe.
2 Vitrectomy: removal of vitreous, hence reducing tractional forces. The vitreous may be replaced with silicone oil or with gas (for example air, SF6, C3F8) in order to tamponade the break.
See below for the pathology of retinal detachment surgery.
Macroscopic
The most common rhegmatogenous detachment specimen presented to the pathologist would be a globe following
multiple failed reattachment procedures that was enucleated for secondary complications such as neovascular glaucoma (Figure 10.103).
Microscopic
It is important to appreciate that the retina commonly detaches by artefact and this is recognised by attachment of photoreceptor fragments to the RPE (Figure 10.104). A pre-existing retinal detachment can be recognised by the presence of photoreceptor atrophy and a reduction in the number of nuclei in the outer nuclear layer (Figures 10.102, 10.104). The photoreceptor layer will not recover after detachment of longer than 6 weeks, and there is a strong case to be made for early surgical intervention. As the detachment progresses over months and years, the retina becomes contracted with a linear appearance (Figure 10.105). At the end stage, the detached retina shows ischaemic atrophy, gliosis, and cyst formation (Figure 10.106). Evidence of neovascular glaucoma may be present.
Pathology of retinal detachment treatment
Conventional surgery The principle of conventional surgery is to close the retinal break by placing the RPE in direct contact with the sensory retina and to relieve the vitreous traction.
The explant is easily recognised macroscopically and may be either localised (radial and segmental circumferential) or encircling (Figure 10.107). Different materials have been used including sponge (Figures 10.107, 10.108) and solid silicone (Figures 10.108, 10.109). It is important to note that the explant is removed prior to paraffin sectioning; the site of the explant appears as an oval space forming an indentation in the sclera (Figure 10.110).
retinal cysts thickened gliotic |
aphakia |
retina |
|
total retinal detachment (tabletop)
Retina - Detachment
Rhegmatogenous (longstanding)
Figure 10.103
Figure 10.103 This globe had an uncomplicated intracapsular lens extraction which was later complicated by retinal detachment. The patient did not report his visual loss until several years later when he presented with neovascular glaucoma. The retina is thickened (by gliosis) and large cysts have formed in the outer retina: this is a common secondary finding in longstanding retinal detachment.
Retina - Detachment
Rhegmatogenous
true rhegmatogenous detachment
artefactual tear in photoreceptors
outer segments attached to RPE
depletion of ONL
atrophic inner
and outer PR segments
Figure 10.104
Figure 10.104 At the edge of a rhegmatogenous detachment, there may be artefactual tearing of the photoreceptors. This appears as a residue of photoreceptor (PR) tips on the retinal pigment epithelium (RPE). After the retina is detached for a period of weeks, the PR inner segments become atrophic and the outer nuclear layer (ONL) is depleted. In this example, there is also atrophy of the inner retina from longstanding glaucoma.
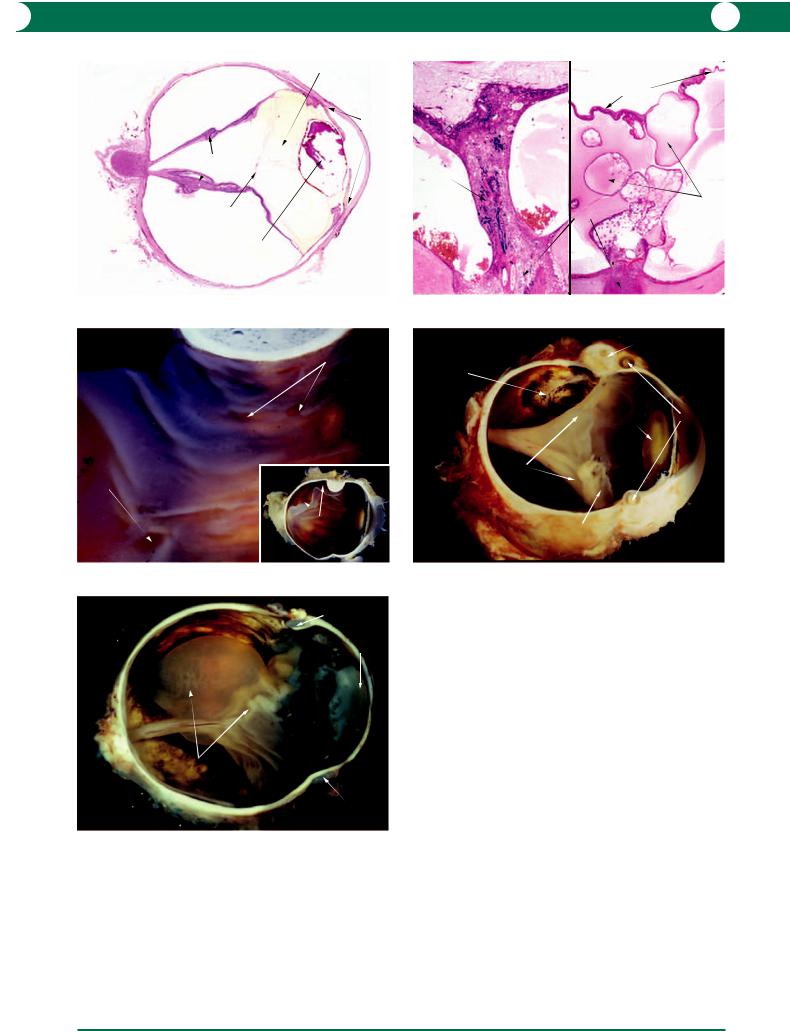
R E T I N A 237
detached retina
posterior vitreous face
artefactual
loss of cataractous lens matter
Retina - Detachment
Rhegmatogenous
Figure 10.105
Retina - Detachment
Rhegmatogenous
Conventional surgery (buckle)
retinal break missed by buckle
collapsed vitreous
closed angles
retinal break in apposition over buckle
retinal detachment
Retina - Detachment
Rhegmatogenous (end stage) atrophic retina
replacement of retinal architecture by glial tissue
optic |
macrocystic |
nerve |
degeneration |
Gliotic |
Cystic |
Figure 10.106 |
|
Retina - Detachment |
segmental |
Rhegmatogenous |
circumferential |
Explant surgery |
explant (sponge) |
cryotherapy |
|
scar |
|
nuclear |
encircling |
cataract |
band |
total |
|
retinal |
|
detachment |
|
(tabletop) |
|
cyst within |
|
retina |
|
Figure 10.107
solid silicone encircling band
collapsed anterior chamber
|
long standing |
|
tabletop retinal detachment |
|
with macrocyst |
Retina - Detachment |
solid silicone |
Rhegmatogenous |
encircling |
Explant surgery |
band |
Figure 10.109
Figure 10.108
Figure 10.105 This archival material illustrates untreated rhegmatogenous retinal detachment. Presumably, the cataractous lens had obscured vision for the patient and the fundus view for the clinician. The retinal detachment was not discovered until secondary angle closure (neovascular glaucoma) had ensued. Note that the optic disc is not cupped; this is a common finding in retinal detachment followed by glaucoma.
Figure 10.106 At the end stage of longstanding detachment, there may be total gliotic replacement of the normal retinal layers (left). Cyst formation (right) may progress to the formation of a macrocyst – see Figure 10.103.
Figure 10.107 An indentation explant consisting of a sponge was used to approximate the retinal pigment epithelium to the retinal break, and to relieve vitreous traction in rhegmatogenous detachment. The breaks are not closed by the explant, suggesting the possibility of pre-existing proliferative vitreoretinopathy (see below). There are also retinal breaks posterior to the explant that are not apposed. The inset shows the complete specimen – the absence of blood in the episcleral tissue is a feature of autopsy material.
Figure 10.108 In this specimen, it may be assumed that the encircling and segmental explants have slipped anteriorly because of the posterior proximity of the cryotherapy burns and the location of the band on the pars plana. The anterior segment structures appear normal apart from a dense nuclear cataract.
Figure 10.109 One complication of an encircling band is that the sclera can be eroded, as in this specimen in which retinal detachment followed surgery for a congenital cataract.

238 C H A P T E R 1 0
Retinopexy – laser photocoagulation and cryotherapy
Current procedures utilise either laser photocoagulation or cryopexy to induce scarring between the retinal break and the underlying RPE in order to seal the retinal defect and promote adhesion (Figures 10.108, 10.111). The appearances of laser photocoagulation are identical to panretinal photocoagulation in ischaemic retinopathy but are on a larger scale. Both types of burn are easily identified as large pale areas of RPE atrophy with patchy hyperpigmentation due to reactionary proliferation of the RPE (Figures 10.48, 10.108, 10.111).
Proliferative vitreoretinopathy (PVR) PVR is the most common cause of failure in retinal detachment surgery. Clinically and pathologically, this is recognised by a distorted, stiffened, and folded retina with or without detachment. A pale membrane with patchy pigmentation may be identified on the inner surface of the retina (Figure 10.112).
This condition is the result of proliferation of glial and RPE cells, usually on the inner retinal surface. RPE cells may also proliferate as strands or cords within the subretinal space (Figure 10.113). The RPE cells undergo metaplasia to fibroblast-like cells with contractile properties. The resultant tissue fibrosis and contracture distorts the inner retina with
further redetachment. The diagnosis of PVR would assume a previous history of either a rhegmatogenous retinal detachment (at least 4–6 weeks postoperative) or a penetrating trauma with access of RPE to the vitreous cavity.
In the enucleated globe, the macroscopic appearance is that of a thickened distorted retina (Figure 10.112). Subretinal strands of proliferating RPE are identified by the presence of retinal folds in the absence of traction (Figure 10.113). Microscopically, the outer retina is atrophic and clumps of RPE cells are present on the inner surface (Figure 10.114, upper). The process continues with fibrous metaplasia of the RPE and the formation of contractile fibrous tissue (Figure 10.114, lower).
If left untreated, tissue contracture from PVR may result in retinal redetachment with formation of new retinal breaks or from reopening of a previous break. Apart from the complications of long term retinal detachment (see above), other features of PVR may include ocular hypotony and shrinkage of the globe (phthisis bulbi).
The pathologist will have previously encountered PVR in globes following unsuccessful retinal detachment surgery complicated by glaucoma. Currently it is more common to receive excised membranes (Figure 10.115) or excised retina removed in the surgical procedure of retinectomy.
Retina - Detachment
Rhegmatogenous / Explant surgery
scar tissue
space enclosing explant
compressed sclera
RPE |
choroid |
Figure 10.110
Retina - Detachment
Proliferative vitreoretinopathy (PVR)
thickened distorted retina
cliliary processes
pigment clumps
fine dusting
of pigmented cells
subretinal exudate
atrophic gliotic retina
fusion between
RPE and retina lymphocytes in
choroid
Retina - Detachment
Rhegmatogenous
Cryotherapy
Figure 10.111
Figure 10.110 Encircling silicone bands are biologically inert. The adjacent scleral tissue is free from inflammatory cell infiltration. In this example, the retina is detached and is not included. A thin strip of choroid lined by proliferating retinal pigment epithelium (RPE) cells underlies the compressed sclera and the encircling band.
Figure 10.111 This is an example of cryotherapy in the treatment of rhegmatogenous retinal detachment. Proliferating retinal pigment epithelium (RPE) cells have migrated into the gliotic retina within the adhesion. Inflammatory cell infiltration is present in the choroid.
Figure 10.112 Proliferation of retinal pigment epithelium cells on the inner surface of the retina is a characteristic feature of proliferative vitreoretinopathy although the extent of the membrane can only be surmised by folds within the detached retina. In this example of end-stage detachment, there is a secondary subretinal exudate and the angles are closed. At this stage it is often impossible to identify a pre-existing break in the peripheral retina.
Figure 10.112

Retina - Detachment
Proliferative vitreoretinopathy (PVR)
subretinal strands of RPE proliferation
shallow retinal detachment
Figure 10.113
Retina - Detachment
Proliferative vitreoretinopathy (PVR) / Excised membrane
metaplastic RPE cells (cuboidal and spindle |
fibrous tissue in vitreous |
|
with intracytoplasmic melanosomes) |
||
|
towards retinal surface
spindle shaped glial cells
Figure 10.115
Retina - Detachment
Tamponading agent
Silicone oil granuloma
NFL of
peripapillary retina intraretinal silicone oil within macrophages
macrophages containing silicone oil globules
Figure 10.117
R E T I N A 239
RPE cells lined by glial cells
|
atrophic PR and ONL Early |
||
proliferating glial cells and |
Late |
||
metaplastic RPE cells |
|||
|
|||
corrugation of ILM |
|
|
|
Retina - Detachment |
|
glial cell |
|
Proliferative vitreoretinopathy (PVR) |
|
proliferation |
|
Figure 10.114 |
|
|
|
Retina - Detachment |
silicone oil globules within macrophages |
||
Tamponading agent |
|||
Silicone oil granuloma |
on the inner surface |
|
|
|
|
||
foamy macrophages phagocytosing lipoprotein in exudate
atrophic outer retina |
atrophic RPE |
|
indicating long term exudative |
||
|
||
retinal detachment |
|
Figure 10.116
Figure 10.113 In this example of a longstanding retinal detachment, the initial appearances are deceptive as the retina appears to be attached. Close observation reveals a shallow retinal detachment. Strands of partially pigmented tissue beneath the retina are indicative of subretinal proliferation of retinal pigment epithelium (RPE). The subretinal location of the ridges is confirmed by the relationship to the overlying retinal vessels.
Figure 10.114 After migration of retinal pigment epithelium (RPE) cells into the vitreous through a retinal break, the cells form clusters on the inner surface of the detached retina (upper). The process continues with spindle cell metaplasia and the formation of fibrous tissue which results in corrugation of the inner limiting membrane (ILM) and contributes to the retinal rigidity (lower).
ONL outer nuclear layer, PR photoreceptors.
Figure 10.115 An excised epiretinal membrane typically consists of metaplastic retinal pigment epithelium (RPE) cells and glial cells embedded in fibrous connective tissue. The RPE cells assume a spindle shape but still possess intracytoplasmic melanosomes.
Figure 10.116 Silicone oil is removed during processing for paraffin histology but the location of the emulsified oil droplets is identified by large empty circular spaces within the epiretinal macrophages. Compare this appearance with the foamy macrophages present in the subretinal exudate. The retina is atrophic due to a longstanding detachment.
Figure 10.117 Emulsified silicone oil can pass through a break in the retina and disperse within the subretinal space. In this example a band of oil laden macrophages is found on the outer surface of the peripapillary retina. The inset shows the presence of oil laden macrophages within the outer part of an atrophic retina – presumably, the outer limiting membrane is less of a barrier to these cells. NFL nerve fibre layer.
