
0647126_DA545_nadeina_l_v_radioekologiya
.pdf
|
{+} |
Radioecology is |
{+} |
Radioecology |
|
|
|
|
|||
|
being |
studying this |
was |
being study- |
|
|
|
|
|||
|
semester. |
ing last semester. |
|
|
|
|
|||||
|
{?} |
Is |
radioecology |
{?} |
Was |
radio- |
|
|
|
|
|
|
being |
studying this |
ecology |
being |
|
|
|
|
|||
|
semester? |
studying |
last |
se- |
|
|
|
|
|||
|
{–} |
Radioecology is |
mester? |
|
|
|
|
|
|
||
|
not |
being studying |
{–} |
Radioecology |
|
|
|
|
|||
|
this semester. |
was |
not |
being |
|
|
|
|
|||
|
|
|
|
studying |
last |
se- |
|
|
|
|
|
|
|
|
|
mester. |
|
|
|
|
|
|
|
Perfect |
Have (has) been + |
Had been + Part |
Shall (will) have |
||||||||
(to have been + |
Part II (ed, V3 ) |
II (ed, V3 ) |
|
been |
+ |
Part II |
|||||
Part II) |
________________ |
______________ |
(ed, V3 ) |
|
|
||||||
|
______________ |
||||||||||
|
{+} |
|
Radioecology |
{+} |
Radioecology |
{+} |
Radioecology |
||||
|
has already been stu- |
had |
been |
studied |
will |
have |
been |
||||
|
died. |
|
by |
December |
last |
studied |
by |
next |
|||
|
{?} Has radioecology |
year. |
|
|
year. |
|
|
|
|||
|
been studied yet? |
{?} |
Had |
radio- |
{?} |
Will |
radio- |
||||
|
{–} |
|
Radioecology |
ecology been stud- |
ecology have been |
||||||
|
has not been studied |
ied? |
|
|
studied? |
|
|
||||
|
yet. |
|
|
{–} |
Radioecology |
{–} |
Radioecology |
||||
|
|
|
|
had not been stud- |
will not have been |
||||||
|
|
|
|
ied. |
|
|
|
studied. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
11
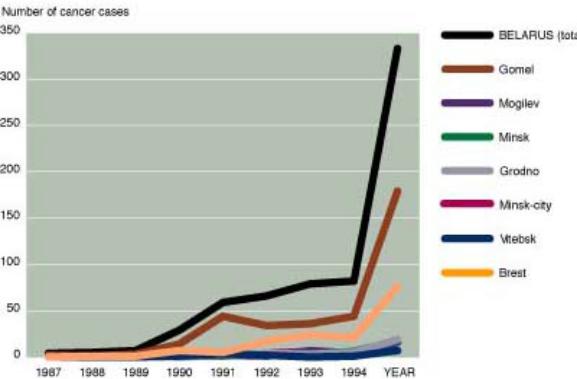
6. Read the text «The Chernobyl accident» and in brackets choose the correct form of the verb in Passive Voice. (See the table «PassiveVoice», p. 9).
The Chernobyl accident
The increase of thyroid cancers among children in various districts of Belarus.
Early in the morning on 26 April 1986 at 1.24 local time a very severe accident occurred at the fourth unit of the Chernobyl nuclear power plant in the former Soviet Union. The operators made some serious errors which resulted in a drastic increase of the effect and ended up in two explosions. The buildings (had been severely damaged /were severely damaged) and fuel fragments and burning graphite blocks (were found / had been found) outside them. Material from the reactor core, including large amounts of radioactive material and smoke from the fire, (are ejected / were ejected) vertically several kilometers into the air. The reactor core was open to the air and in the following days sealing materials (were being dumped / were dumped) into the core. However, this resulted in increased core temperature and increased release of radioactive material. It was not until May 5 that they managed to cool the reactor with liquid nitrogen and the release of radioac-
12
tive material (could be stopped / have been stopped or at least be reduced / have been reduced) to a low amount. The unit 4 core contained an inventory of 4x1019 Bq and it (is being estimated / has been estimated) that at least 1x1018 to 2x1018 Bq was released. During these 10 days, a large part of the inventory of radionuclides of the reactor (had been spread out / was spread out) not only over a part of the Soviet Union but also over most of the countries in Europe and detectable amounts over nearly all parts of the northern hemisphere. One of the biggest accidents in the world had occurred.
After the two explosions, large amounts of burning material (had been spread out / was spread out) around the buildings and fires occurred on several sites. Shortly after the accident, three fire-fighting teams arrived and augmented the teams at the site. The main challenges were to prevent the fire from spreading to unit 3 since the roof of the machine hall shared by units 3 and 4 was burning. The fire-fighters did not have appropriate protective clothing and they received quite high radiation doses, both whole-body irradiation due to the high external dose rate and also high beta contamination that caused severe beta skin burns. Many of them suffered from acute radiation sickness, with vomiting and nausea already during the fire-fighting. Of the on-site personnel, about 300 (were being hospitalized / were hospitalized) due to acute radiation syndromes and burns. Of these, 28 died due to acute radiation injuries.
On April 27, the 45,000 inhabitants in Pripiat, the nearest town, (had been evacuated / were evacuated), followed some days later by all 135,000 people living within a radius of 30 km. Probably none of them will return and live within the 30 km zone. In addition to the people, some tens of thousands of cattle (were being evacuated / were also evacuated) from the 30 km zone.
(http://www.envimed.com/emb08.shtml)
13
UNIT II
History of Radioactivity
Warming-up
Look at the portraits of these well-known scientists.
 A
A
 B
B
14
Read the information about these scientists match the letters with the numbers and answer the questions.
1)He was born into a family of scientists. His grandfather had made important contributions in the field of electrochemistry while his father had investigated the phenomena of fluorescence and phosphorescence. He not only inherited their interest in science, he also inherited the minerals and compounds studied by his father. The material he chose to work with was potassium uranyl sulfate, K2UO2(SO4)2, which he exposed to sunlight and placed on photographic plates wrapped in black paper. When developed, the plates revealed an image of the uranium crystals. He concluded «that the phosphorescent substance in question emits radiation which penetrates paper opaque to light». Initially he believed that the sun's energy was being absorbed by the uranium which then emitted X-rays. Further investigation, on the 26th and 27th of February, was delayed because the skies over Paris were overcast and the uranium-covered plates he intended to expose to the sun were returned to a drawer. On the first of March, he developed the photographic plates expecting only faint images to appear. To his surprise, the images were clear and strong. This meant that the uranium emitted radiation without an external source of energy such as the sun. He had discovered radioactivity, the spontaneous emission of radiation by a material.
Later, he demonstrated that the radiation emitted by uranium shared certain characteristics with X-rays but, unlike X-rays, could be deflected by a magnetic field and therefore must consist of charged particles. For his discovery of radioactivity, he was awarded the 1903 Nobel Prize for physics.
2)He was a Professor at Wuerzburg University in Germany. Working with a cathode-ray tube in his laboratory, he observed a fluorescent glow of crystals on a table near his tube. The tube that he was working with consisted of a glass envelope (bulb) with positive and negative electrodes encapsulated in it. The air in the tube was evacuated, and when a high voltage was applied, the tube produced a fluorescent glow. He shielded the tube with heavy black paper, and discovered a green colored fluorescent light generated by a material located a few feet away from the tube. He concluded that a new type of ray was being emitted from the tube. This ray was capable of passing through the heavy paper covering and exciting the phosphorescent materials in the room. He found that the new ray could pass through most substances casting shadows of solid objects. He also discovered that the ray could pass through the tissue of humans, but not bones and metal objects.
(www:history.htm)
15
1.Can you name these scientists?
2.Were their researches connected with the topic of this unit?
3.Whose film of hand do you think it was?
1. Do you know that …?
History of the Radioactivity Warning Symbol
Did you know the original radiation warning symbol that was devised in 1946 at that University of California, Berkeley Radiation Laboratory was magenta on a blue background?
The colours were changed to a black trefoil on a yellow background,
with specific attributes regarding the relative diameter of the blades and their orientation.
The central circle has radius R, with the inner section extending for a radius of 1.5 R.
The fan blades are 5 R, separated from each other by 60°. (www:History of the Radioactivity warning symbol-Did you know.htm)
16

1.1 This is a collection of radiation warning symbols and radioactivity warning signs. What do the symbols and signs warn about?
I
A B C D
E F G H
L
I J K
1.2 Match the letters with the numbers.
1)This radiation symbol is a little fancier than your standard trefoil, but it is easy to recognize the significance of the symbol.
2)This is the warning symbol for non-ionizing radiation.
3)This symbol warns of a radiation hazard.
4)This symbol warns of the risk of exposure to laser beams or coherent radiation.
5)This sign warns of a radiation hazard.
6)The original radiation warning symbol was devised in 1946 at the University of California, Berkeley Radiation Laboratory. Unlike the modern black on yellow symbol, the original radiation symbol featured a magenta trefoil on a blue background.
17
7)This trefoil is the hazard symbol for radioactive material.
8)This symbol indicates the presence of an optical radiation hazard.
9)The radiation symbol warning of an ionizing radiation hazard.
10)This is the US Army symbol for a radiation WDM or nuclear weapon.
11)This sign warns of laser radiation.
12)This is the IAEA ionizing radiation warning symbol.
2. Read the text and for questions 1-9 choose the best answer: A, B or C.
Three Types of Radioactivity
There are three types of radioactivity. Gamma rays come from the nucleus of the atom of a radioactive isotope. They are the most energetic and most penetrating of all radiation. Some radiation travel as particles not waves and is also emitted by the radioactive isotope. One is alpha particles that lose energy quickly. A hand or thin piece of paper stops it. Beta particles are high speed electrons that travel close to the speed of light and can penetrate a hand but not concrete.
When an atom undergoes radioactive decay, it emits one or more forms of radiation with sufficient energy to ionize the atoms with which it interacts. Ionizing radiation can consist of high speed subatomic particles ejected from the nucleus or electromagnetic radiation (gamma-rays) emitted by either the nucleus or orbital electrons.
Alpha Particles
Certain radionuclides of high atomic mass (Ra226, U238, Pu239) decay by the emission of alpha particles. These alpha particles are tightly bound units of two neutrons and two protons each (He4 nucleus) and have a positive charge. Emission of an alpha particle from the nucleus results in a decrease of two units of atomic number (Z) and four units of mass number (A). Alpha particles are emitted with discrete energies characteristic of the particular transformation from which they originate. All alpha particles from a particular radionuclide transformation will have identical energies.
18

Beta Particles
A nucleus with an unstable ratio of neutrons to protons may decay through the emission of a high speed electron called a beta particle. This results in a net change of one unit of atomic number (Z). Beta particles have a negative charge and the beta particles emitted by a specific radionuclide will range in energy from near zero up to a maximum value, which is characteristic of the particular transformation.
Gamma-rays
A nucleus which is in an excited state may emit one or more photons (packets of electromagnetic radiation) of discrete energies. The emission of gamma rays does not alter the number of protons or neutrons in the nucleus but instead has the effect of moving the nucleus from a higher to a lower energy state (unstable to stable). Gamma ray emission frequently follows beta decay, alpha decay, and other nuclear decay processes.
(www:radiography/physics/gamma.htm)
19
1. They are the most energetic and most penetrating of all radiation.
|
A) Alpha particles |
B) Gamma rays |
C) Beta particles |
2. |
They are emitted with discrete energies characteristic of the particular |
||
transformation from which they originate. |
|
||
|
A) Alpha particles |
B) Gamma rays |
C) Beta particles |
3. |
They have a negative charge and they emitted by a specific radionuclide |
||
will range in energy from near zero up to a maximum value, which is charac-
teristic of the particular transformation. |
|
||
|
A) Alpha particles |
B) Gamma rays |
C) Beta particles |
4. |
Gamma rays come from the nucleus of the atom of a radioactive isotope. |
||
|
A) Alpha particles |
B) Gamma rays |
C) Beta particles |
5. |
They are tightly bound units of two neutrons and two protons each and |
||
have a positive charge. |
|
|
|
|
A) Alpha particles |
B) Gamma rays |
C) Beta particles |
6. |
A hand or thin piece of paper stops them. |
|
|
|
A) Alpha particles |
B) Gamma rays |
C) Beta particles |
7. All of them from a particular radionuclide transformation will have identi-
cal energies. |
|
A) Alpha particles B) Gamma rays |
C) Beta particles |
8. The emission of them does not alter the number of protons or neutrons in the nucleus but instead has the effect of moving the nucleus from a higher to
a lower energy state. |
|
A) Alpha particles B) Gamma rays |
C) Beta particles |
9. They are high speed electrons that travel close to the speed of light and
can penetrate a hand but not concrete. |
|
A) Alpha particles B) Gamma rays |
C) Beta particles |
3. Read the text «Radiation» paying attention to the terms in bold.
especially – особенно; главным образом by contrast – по контрасту
20
