
0647126_DA545_nadeina_l_v_radioekologiya
.pdf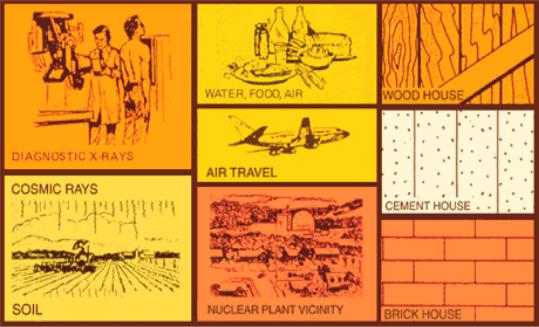
i.e – то есть
instead of – вместо чего-либо; взамен чего-л. nevertheless – тем не менее; однако, все-таки either …. or – или ….. или
in the same way – так же because – потому что; так как
however – однако; тем не менее; несмотря на (э)то for example – например
according to – в соответствии с; согласно; по more broadly – значительно шире; более широко thus – так; таким образом; итак; соответственно in addition – к тому же; кроме того; сверх того
Radiation
Radiation is energy in motion. Not only does radiation come from elements in the form of radioactivity, some come from our natural environment, others from human activities and vices.
21
Non-ionizing radiation: Non-ionizing radiation, by contrast, refers to any type of radiation that does not carry enough energy per photon to ionize atoms or molecules. Most especially, it refers to the lower energy forms of electromagnetic radiation (i.e., radio waves, microwaves, terahertz radiation, infrared light, and visible light). The effects of these forms of radiation on living tissue have only recently been studied. Instead of producing charged ions when passing through matter, the electromagnetic radiation has sufficient energy only for excitation, the movement of an electron to a higher energy state. Nevertheless, different biological effects are observed for different types of non-ionizing radiation.
Neutron radiation: Neutron radiation is a kind of non-ionizing radiation that consists of free neutrons. These neutrons may be emitted during either spontaneous or induced nuclear fission, nuclear fusion processes, or from other nuclear reactions. It does not ionize atoms in the same way that charged particles such as protons and electrons do (exciting an electron), because neutrons have no charge. However, neutron interactions are largely ionizing, for example when neutron absorption results in gamma emission and the gamma subsequently removes an electron from an atom, or a nucleus recoiling from a neutron interaction is ionized and causes more traditional subsequent ionization in other atoms.
Electromagnetic radiation: Electromagnetic radiation (sometimes abbreviated EMR) takes the form of self-propagating waves in a vacuum or in matter. EM radiation has an electric and magnetic field component which oscillate in phase perpendicular to each other and to the direction of energy propagation. Electromagnetic radiation is classified into types according to the frequency of the wave, these types include (in order of increasing frequency): radio waves, microwaves, terahertz radiation, infrared radiation, visible light, ultraviolet radiation, X-rays and gamma rays. Of these, radio waves have the longest wavelengths and Gamma rays have the shortest. A small window of frequencies, called visible spectrum or light, is sensed by the eye of various organisms, with variations of the limits of this narrow spectrum. EM radiation carries energy and momentum, which may be imparted when it interacts with matter. The electromagnetic spectrum is the range of all possible electromagnetic radiation frequencies. The electromagnetic spectrum of an object is the characteristic distribution of electromagnetic radiation emitted by, or absorbed by, that particular object.
Light: Light, or visible light, is electromagnetic radiation of a wavelength that is visible to the human eye (about 400–700 nm), or up to
22
380–750 nm. More broadly, physicists refer to light as electromagnetic radiation of all wavelengths, whether visible or not.
Thermal radiation: Thermal radiation is the process by which the surface of an object radiates its thermal energy in the form of electromagnetic waves. Infrared radiation from a common household radiator or electric heater is an example of thermal radiation, as is the light emitted by a glowing incandescent light bulb. Thermal radiation is generated when heat from the movement of charged particles within atoms is converted to electromagnetic radiation. The emitted wave frequency of the thermal radiation is a probability distribution depending only on temperature and for a genuine black body is given by Planck’s law of radiation. Wien's law gives the most likely frequency of the emitted radiation, and the Stefan–Boltzmann law gives the heat intensity.
Black-body radiation: Black-body radiation is a common synonym for thermal radiation (see above). It is so-called because the ideal radiator of thermal energy would also be an ideal absorber of thermal energy: It would not reflect any light, and thus would appear to be absolutely black.
In addition, people are exposed to radiation from man-made sources such as color televisions, smoke detectors, computer monitors, and X-rays. These sources account for less than one-fifth of our total radiation exposure. There is no difference between natural radiation and its effects and manmade radiation and its effects.
Cosmic radiation and air travel: Cosmic radiation is part of our natural environment, and we are constantly exposed to a certain amount of ionizing radiation. Radiation originating from outer space and the sun is called cosmic radiation and contributes about 13% of the background radiation level on Earth (a greater part is due to radon).
Cosmic radiation is a collection of many different types of radiation from many different types of sources. When people speak simply of 'cosmic radiation' they are usually referring specifically to the cosmic microwave background radiation. This consists of very, very low energy photons (energy of about 2.78 Kelvin) whose spectrum is peaked in the microwave region and which are remnants from the time when the universe was only about 200,000 years old.
23

There are also very old remnant neutrinos in the cosmic radiation. Neutrinos pass through just about everything with no effect so they are harmless. The photons are too low in energy to be dangerous. On top of these there are higher energy particles that are being created constantly by all luminous objects in the universe. Photons of all different energies/wavelengths are being created by our sun, other stars, quasi-stellar objects, black-hole accretion disks, gamma-ray bursts and so on. These objects also produce high-energy massive particles such as electrons, muons, protons and anti-protons. These higher energy particles are potentially dangerous, but most of these particles never make it to the earth. They are deflected by magnetic fields between us and the source, or they interact with other particles, or they decay in flight.
The particles that do make it to the earth interact with our atmosphere, which acts as a «radiation shield». The high-energy cosmic rays bombard us all the time, but they interact quickly, producing particles of much lower energy which impact the earth harmlessly. If this was dangerous to us, we wouldn't be here to discuss these things! Some particles, like neutrinos and
24
high energy muons, are passing through us all the time, but they interact so weakly that they have no effect on our bodies. Of course, if we were in space without the protection of our atmosphere then we would need some other type of shielding from the radiation (spacesuits and protective covering on our spacecrafts).
The radiation to worry about, of course, is the 'cosmic' radiation produced by our sun. There is only one type of cosmic radiation known to adversely affect us and that's UV radiation from our sun, which causes skin cancer in millions of people every year. Again, our atmosphere serves as a shield, but ultraviolet photons do make it through – and without that protective ozone layer which blocks these photons we're all going to need a lot more sunscreen!
Special features of cosmic radiation: Cosmic radiation is a complex mixture of charged and neutral particles, some of them generated when primary particles from space interact with the earth's atmosphere. This complexity also leads to difficulties in measuring radiation doses from cosmic radiation, but physicists have developed sophisticated approaches to deal with this situation. For the human exposure situation one feature of cosmic radiation is of particular importance: a large percentage of the effective radiation dose from cosmic radiation is due to neutrons of different energy levels. Neutrons are subatomic particles which - when compared to X-rays or Gamma rays - cause more biological damage per dose unit.
Exposure during flying: As a rule, cosmic radiation levels rise with increasing altitude (up to about 20 km above ground). The actual radiation level is influenced by a number of factors, most importantly through the shielding provided by the earth's atmosphere. The overall effect for flight crew and travelers is an increased radiation exposure during flights as compared to staying on the ground. Flight crew passes up to 1000 hours per year on board of flying planes, which leads to annual effective radiation doses in the range of 2 to 5 milliSievert (mSv) for most crew. Occasional travelers obtain a fraction of this value through less frequent leisure or occupational flights. In comparison, the natural background radiation amounts to 2 to 3 mSv per year at most geographical locations worldwide.
(Merril Eisenbud, Thomas F.Gesell, Environmental radioactivity: from natural, industrial, and military sources. Academic Press, 1997)
25
3.1 Give the English equivalents to the Russian words:
1.The effects of (этих форм радиации) on living tissue have only recently been studied.
2.It does not ionize atoms in the same way that (заряженные частицы) (такие как) protons and electrons do (exciting an electron), because neutrons have no charge.
3.A small window of frequencies, called (видимый спектр) or light, (улавливается) by the eye of various organisms, with variations of the limits of this narrow spectrum.
4.Thermal radiation is generated when heat from the (движение заряженных частиц) within atoms (преобразовывается) to electromagnetic radiation.
5.These sources account for (менее 1/5) of our total radiation exposure.
6.Neutrinos (проходят почти через все) with no effect so they are harmless.
7.These higher energy particles are (потенциально опасны), but most of these particles never make it to the earth.
8.The high-energy cosmic rays bombard us all the time, but they (быстро взаимодействуют), producing particles of much lower energy which impact the earth harmlessly.
9.The radiation (которая нас беспокоит), of course, is the 'cosmic' radiation produced by our sun.
10.Again, our atmosphere serves (как щит), but ultraviolet photons do make it through – and without that protective (озоновый слой) which blocks these photons we're all going to need a lot more sunscreen.
11.This (сложность) also leads to (трудностям) in (измерении доз радиации) from cosmic radiation, but physicists have developed sophisticated approaches to deal with this situation.
12.The actual radiation level is influenced by (ряд факторов), most importantly through the shielding provided by (атмосфера Земли).
13.Cosmic radiation is a (сложная смесь заряженных и) neutral particles, some of them generated when primary particles from space interact with the earth's atmosphere.
14.Of course, (если бы мы были в космосе) without the protection of our atmosphere then we would need some other type of shielding from the radiation (spacesuits and protective covering on our spacecrafts).
15.(Если бы это было опасно для нас), we wouldn't be here to discuss these things!
26

16. (Существует только один известный тип космической радиации) to adversely affect us and that's UV radiation from our sun, which causes skin cancer in millions of people every year.
4. Read the text.
Isotopes
Isotopes are used in modern medicine for research purposes and diagnose diseases. In this picture a researcher is working with a radioactive isotope in a laboratory. A number of precautions are required to protect the person from too large a dose of the radioactive material.
Isotopes in Research and Medicine:
Scientists can now create radioactive forms of common elements, called isotopes. Each isotope has a fixed rate of decay which can be characterized by its half-life, or the length of time that it takes half of the radioactive atoms in a sample to decay. Because each isotope decays at a unique and predictable rate, different isotopes can be used for a variety of purposes. For example, isotopes play an important role in modern medicine. They can be ingested and traced in their path through the body, revealing biochemical and metabolic processes with precision. These isotropic «tracers» are currently used for practical diagnosis of disease as well as in research.
The dating of radioactive carbon has helped to define the history of life on this planet. Any living organism takes in both radioactive and nonradioactive carbon, either through the process of photosynthesis or by eating plants or eating animals that have eaten plants. When the animal dies, however, uptake of carbon stops. As a result, radioactive carbon atoms are not replaced as they decay, and the amount of this material decreases over time.
27
The rate of decrease is predictable and can be described with accuracy, vastly increasing our ability to date the biological events of our planet.
4.1 Memorize the following pairs of derivatives.
Noun |
Adjective |
radioactivity |
radioactive |
energy |
energetic |
orbit |
orbital |
atom |
atomic |
nature |
natural |
biology |
biological |
difference |
different |
tradition |
traditional |
electricity |
electric |
magnet |
magnetic |
length |
long |
vision |
visible |
variety |
various |
possibility |
possible |
character |
characteristic |
frequency |
frequent |
danger |
dangerous |
mass |
massive |
protection |
protective |
complexity |
complex |
difficulty |
difficult |
importance |
important |
geography |
geographical |
prediction |
predictable |
practice |
practical |
4.2 Choose between the alternatives to complete these sentences.
1. The electromagnetic spectrum of an object is the characteristic/character distribution of electromagnetic radiation emitted by, or absorbed by, that particular object.
28
2.For the human exposure situation one feature of cosmic radiation is of particular important/importance: a large percentage of the effective radiation dose from cosmic radiation is due to neutrons of different energy levels.
3.The photons are too low in energetic/energy to be dangerous.
4.Cosmic radiation is a collection of many difference/different types of radiation from many different/difference types of sources.
5.Electromagnetic radiation is classified into types according to the frequent/frequency of the wave, these types include: radio waves, microwaves, terahertz radiation, infrared radiation, visible/vision light, ultraviolet radiation, X-rays and gamma rays.
6.Ionizing radiation can consist of high speed subatomic particles ejected from the nucleus or electromagnetic radiation (gamma-rays) emitted by either the nucleus or orbital/orbit electrons.
7.Neutron interactions are largely ionizing, for example when neutron absorption results in gamma emission and the gamma subsequently removes an electron from an atomic/atom or a nucleus recoiling from a neutron interaction is ionized and causes more traditional/tradition subsequent ionization in other atoms.
8.These objects also produce high-energy massive/mass particles such as electrons, muons, protons and anti-protons.
9.These isotropic "tracers" are currently used for practical/practice diagnosis of disease as well as in research.
10.Not only does radiation come from elements in the form of radioactive/radioactivity, some come from our natural environment, others from human activities and vices.
5.Choose the right preposition to the following verbs. Some prepositions can be used twice.
1. to consist |
|
a) with |
2. to result |
|
b) from |
3. to come |
|
c) on |
4. to refer |
|
d) into |
5. to pass |
|
e) to |
6. to be classified |
|
f) through |
7. to interact |
|
g) in |
8. to depend |
|
h) of |
9. to originate |
|
j) up |
10. to deal |
|
|
|
29 |
|
5.1 Put a preposition in each of the numbered spaces.
1.Most of the particles passed 1) _____ the foil undisturbed, suggesting that the foil was made up mostly of empty space rather than of a sheet of solid atoms.
2.Atomic particles, Rutherford's work showed, consisted 2) ___ empty space surrounding a well-defined central core called a nucleus.
3.As they pass 3) _____ matter, they are scattered and absorbed and the degree of penetration depends 4) ___ the kind of matter and the energy of the rays.
4.A single interaction event between a primary x-ray photon and a particle of matter does not usually result 5) ____ the photon changing to some other form of energy and effectively disappearing.
5.The event is also known as incoherent scattering because the photon energy change resulting 6) _____ an interaction is not always orderly and consistent.
6.The energy shift depends 7) ___ the angle of scattering and not on the nature of the scattering medium.
7.Radioecology is a branch of ecology which studies how radioactive substances interact 8) ____ nature, how different mechanisms affect the substances migration and uptake in food chain and ecosystems.
8.Fallout commonly refers 9) ____ the radioactive dust created when a nuclear weapon explodes.
9.Radioecology is a science that came 10) ___ after the first tests of nuclear bombs.
10.The radiation sickness is generally used to refer 11) ___ acute problems caused by a large dosage of radiation in a short period, though this also has occurred with long term exposure.
6. Read the text and do the task.
The Discovery of Radioactivity:
One hundred years ago, a group of scientists unknowingly ushered in the Atomic Age. Driven by curiosity, these men and women explored the nature and functioning of atoms. Their work initiated paths of research which changed our understanding of the building blocks of matter; their discoveries prepared the way for development of new methods and tools used to explore our origins, the functioning of our bodies both in sickness and in health, and much more.
30
