
02 BOPs / Woods D.R 2008 rules-of-thumb-in-Engineering-practice (epdf.tips)
.pdf
6.1 Factors Affecting the Choice of Reactor 191
Figure 6.2 Pressure–temperature operating window.
Table 6.6 illustrates the impact of the reaction mechanism on the choice of reactor. The important parameters are the order of the reaction, the reaction rate and the activation energy, E. Models for the reaction rate, for an example reaction A + B = P, are of the form –rate = k cAa1 cBb1. The activation energy, E, is usually represented as the Arrhenius number = E/RT and k = exp (Arr). In general, E = 20–30 MJ/kmol for polymerizations; 150–250 MJ/kmol for uncatalyzed gaseous decompositions. The temperature dependence of the reaction rate is directly related to the activation energy. If the reaction temperature is about 300 hC, then the reaction rate doubles for a temperature increase of 45 hC (if E = 50 MJ/ kmol), an increase of 12 hC (if E = 150 MJ/kmol) and 6 hC (if E = 300 MJ/kmol).
All these factors lead to the central issue of selecting the type and configuration of the reactor (the central issue in Fig. 6.1). That choice converts into costs as we identify the processing conditions and configurations and the required ancillary equipment. If a catalyst has been selected, then the reaction cycle and the catalyst replacement/regeneration become important. If a batch or semi-batch reactor is selected, then the cycle times become issues.
Section 6.2 provides rules of thumb that help to quantify the decisions on some of the major issues illustrated on this sizing map.

|
192 |
6 |
Reactors |
|
|
|
|
|
|
|
|
|
|
||||
Table 6.6 Selecting reactor configuration based on the type of reactions. |
|
|
||||||
|
|
|
|
|
|
|||
Type of reaction |
|
|
|
Reactor |
Operating conditions |
|||
|
|
|
|
|
|
|
||
Single Reactions: |
|
|
|
|
|
|
||
|
|
|
|
|
||||
Reversible; equili- |
|
maximize selectivity |
ideal batch or |
use excess of feed that is less ha- |
||||
brium |
|
|
|
assume 95 % equilib. |
PFTR ; |
zardous and easiest to separate |
||
A m P |
c a |
|
|
conversion |
|
series of adiabatic |
and remove the product stagewise |
|
|
|
|
|
|
|
beds if exothermic |
|
|
|
|
|
|
|
|
|||
A + B m P + Waste |
|
|
use excess of |
if n product |
||||
|
|
|
|
|
|
|
feed that is less |
i n reactants |
|
|
|
|
|
|
|
hazardous and |
add inerts |
|
|
|
|
|
|
|
easiest to sepa- |
|
|
|
|
|
|
|
|
rate |
|
|
|
|
|
|
|
|
|
if n product |
|
|
|
|
|
|
|
|
I n reactants no |
|
|
|
|
|
|
|
|
inerts to feed |
|
|
|
|
|
|
|
||
A + B p P |
k1 c a1 |
|
PFTR |
isothermal |
|
|||
|
|
|
|
|
|
CSTR + PFTR |
adiabatic or PFTR if the reaction |
|
|
|
|
|
|
|
|
rate decreases monotonically with |
|
|
|
|
|
|
|
|
conversion |
|
|
|
|
|
|
|
|
||
A p P |
k1 c a1 |
|
minimize volume |
ideal batch or |
|
|
||
|
|
|
|
assume 95 % conversion |
PFTR |
|
|
|
|
|
|
|
|
|
|||
Multiple parallel (simultaneous parallel) |
|
|
|
|
||||
|
|
|
|
|
|
|||
A p P |
k1 c a1 |
|
a2 i a1 selectivity increases |
CSTR |
keep cA low; use large recycle, low |
|||
|
|
|
|
assume 95 % conversion |
|
pressure and add inerts but the |
||
A p W |
k2 c a2 |
|
|
|
|
rate is lower. |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
a2 I a1 selectivity decreases |
Ideal batch or |
keep cA high; no recycle, high |
||
|
|
|
|
assume 50 % conversion |
PFTR |
pressure and no inerts |
||
|
|
|
|
|
|
|
||
A + B _p P |
|
|
a2 i a1 |
b2 i b1 |
CSTR |
If E1 i E2 use high temperature |
||
k1 c a1 c b1 |
|
|
|
|
|
If E1I E2 use low temperature |
||
|
|
|
|
|
|
|
Where E = activation energy for |
|
A + B p W |
|
|
|
|
|
the reaction. |
|
|
k2 c a2 c b2 |
|
|
|
|
|
To maximize yield, use low tem- |
||
|
|
|
|
|
|
|
perature initially and then in- |
|
|
|
|
|
|
|
|
crease the temperature as the rate |
|
|
|
|
|
|
|
|
diminishes; use high initial con- |
|
|
|
|
|
|
|
|
centration of one of the feeds. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
b2 I b1 |
semi batch or |
|
|
(continued) |
|
|
|
|
cold shot PFTR |
(continued) |
|
|
|
|
|
|
|
|
|
|
|

6.1 Factors Affecting the Choice of Reactor 193
Table 6.6 Continued.
Type of reaction |
|
|
|
Reactor |
Operating conditions |
|
|
|
|
|
|
(as previous) |
a2 I a1 |
b2 |
i b1 |
batch PFTR; |
(as previous) |
|
|
|
|
series CSTR |
|
|
|
|
|
|
|
|
|
b2 |
I b1 |
batch PFTR; |
|
|
|
|
|
series CSTR |
|
Multiple series or sequential (e.g. polymerization, degradation, crystallization)
A p P |
|
|
assume 50 % conversion |
batch or |
If E1 i E2 use high temperature |
|
|
|
|
|
|
PTFR |
|
P p W |
|
|
|
|
|
|
|
|
|
|
|
|
If E1 I E2 use high temperature |
|
|
|
|
|
|
initially to produce product ra- |
|
|
|
|
|
|
pidly and then decrease tempera- |
|
|
|
|
|
|
ture as product accumulates. |
|
|
|
|
|||
Mixed parallel and series |
|
|
||||
|
|
|
|
|
|
|
A p P |
k1 c a1 |
a2 i a1 |
Combo CSTR + |
|
||
|
|
|
|
|
PFTR |
|
A p W k2 c a2 |
|
|
PFTR + CSTR |
|
||
|
|
|
|
|
PFTR with large |
|
P p W 2 k3 c a3 |
|
|
recycle |
|
||
|
|
|
|
|
series of CSTR |
|
|
|
|
|
|
|
|
|
|
|
a2 I a1 |
PTFR |
|
|
|
|
|
|
|
||
A + B p P |
k1 c a1 |
c b1 |
|
For E3 i E1 i E2 search for best |
||
|
|
|
|
|
|
intermediate feed temperature. |
P p W1 |
k2 c a2 |
|
|
|
|
|
P p W2 |
k3 c a3 |
|
|
|
|
|
|
|
|
|
|||
A + B p P |
k1 c a1 c b1 |
|
For E3 i E1 i E2 search for best |
|||
|
|
|
|
|
|
intermediate feed temperature. |
A + B p W1 k2 c a2 c b2 |
|
|
||||
A + B p W2 k3 c a3 c b3

194 |
6 Reactors |
|
|
|
|
|
|
|
|
Table 6.6 Continued. |
|
|
||
|
|
|
|
|
Type of reaction |
Reactor |
Operating conditions |
||
|
|
|
|
|
[1] A + B p Inter- |
|
E1 i E2 and E3 i E4 start with |
||
mediate |
|
|
|
high temperature and reduce. |
k1 c a1 c b1 |
|
|
|
|
|
|
|
|
E1 I E2 and E3 I E4 start with |
[2] A p W1 |
k2 c a2 |
|
low temperature and increase |
|
[3] Intermediate p W 2 |
|
E1 i E2 and E3 I E4 use maxi- |
||
k3 c a3 |
|
|
|
mum possible temperature |
[4] Intermediate p P |
|
E1 I E2 and E3 i E4 use lowest |
||
k4 c a4 |
|
|
|
possible temperature |
|
|
|
|
|
Polycondensation with |
PFTR or batch |
|
||
no termination reac- |
|
|
||
tion |
|
|
|
|
|
|
|
|
|
Free radical |
|
active polymer life |
PFTR or batch |
difficult if the system is viscous |
|
|
ii residence time |
|
and there is buildup on the walls |
|
|
|
|
that reduces the heat transfer |
|
|
|
|
|
|
|
active polymer life |
CSTR |
|
|
|
II residence time |
|
|
|
|
|
|
|
Copolymers |
|
|
CSTR |
|
|
|
|
|
|
6.2
General Guidelines
Here are some general rules of thumb and specific guidelines about the phase for either gas or liquid phases. Suggestions for reaction using solid catalysts and for gases reacting with solids are given in Sections 6.2.3 and 6.2.4. Bioreactors, reactions under supercritical conditions and polymerizers are discussed in Sections 6.2.5 and 6.2.7, respectively. Section 6.2.8 considers how the phases present guide the choice of reactor.
6.2.1
General Rules of Thumb for the Type of Reactor
Temperature effects: For reactions that are rate or kinetics controlled, at low temperatures and for moderate activation energies, the rate of reaction doubles for a temperature increase of 10 hC. For low activation energies or for temperatures above 500 hC, the rate doubles for a temperature increase of 25 to 35 hC. For reac-

6.2 General Guideline 195
tions in the liquid phase that are mass transfernot kineticallycontrolled, the liquid phase mass transfer increases by two when the temperature increases by 35 hC. For gas liquid, GL, reactions that are mass transfernot kinetically-con- trolled, the solubility decreases by two when the temperature increases by 35 hC and the liquid phase mass transfer increases by two when the temperature increases by 35 hC so the net effect is no change in the rate of reaction with temperature. Figure 6.3 shows how the residence time in a reactor decreases with increase in temperature, if the reaction is kinetically controlled. Other lines show how the liquid phase mass transfer coefficient, the gas solubility and the net result for some gas–liquid reactions change with temperature. A “characteristic trajectory line” is included as a reference guide for information given subsequently in Fig. 6.6.
For exothermic reactions, a temperature increase causes the equilibrium constant to decrease; vice versa for endothermic reactions (Le Chatelier, F).
It is difficult to talk about fast or slow reactions because a reaction may be slow to an organic chemist but fast to a biotechnical specialist. However, in this book I have used the following terms, expressed in units consistent with first order reactions:
Extremely fast reaction, rate i 1000 1/s Very fast, rate 500–1000 1/s
Fast reaction, rate 50–500 1/s Intermediate, reaction rate 2–50 1/s Slow reactions, rate 0.01–2 1/s Very slow, rate 10–4 –10–2 1/s Extremely slow, rate10–6–10–4 1/s Lengthy, rate I 10–6 1/s
|
microseconds |
dehydrogenation of ethyl benzene |
seconds |
liquid phase chlorinations |
minutes |
bromination of xylene |
|
polymerizations |
hours |
fermentations |
days |
To be viable, the rate of production for gaseous catalyzed reactions should be i 3 q 10–4 kmol/s m3 or at 327 hC (or RT = 9.314 q 600) about 1.7 1/s, that is, faster than intermediate.
For reaction rates that are not first order the units are not 1/s. As approximations these can be converted into “first order” reactions so that we can estimate the speed of the reaction in terms of the above definitions. For example, a reaction rate expressed as mol/s g catalyst can be converted into a “first order” rate by multiplying by the bulk density of the catalyst and by RT. Similarly, rates expressed as mol/s m3 can be converted by multiplying by RT.
Most gas phase and liquid phase reactions are kinetically controlled as opposed to being equilibrium controlled (except for the oxidation of sulfur dioxide to sulfur trioxide, the production of methanol from synthesis gas, the production of ammonia from synthesis gas, and the production of hydrogen from the water-gas shift reaction – these are equilibrium controlled).
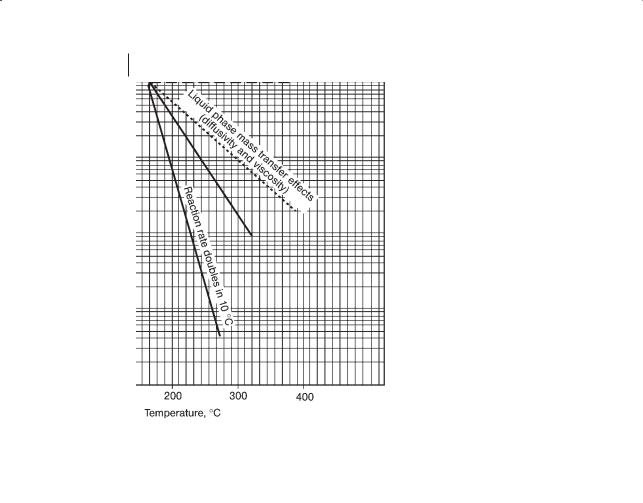
196 6 Reactors
Figure 6.3 Residence time versus temperature.
Most gas–solid reactions are heat transfer controlled.
Most heterogeneous catalytic gas reactions are mass transfer controlled. For slow reaction rates, prefer batch to continuous.
Prefer continuous to semicontinuous to batch (H). Prefer PFTR to CSTR (H).
Prefer minimum inventory in reactor by providing better mixing, increased temperature or pressure or changing the catalyst (H).
Prefer internally heated/cooled to externally heated/cooled (H). Consider light and ultrasound as optional forms of energy (H).
The heat of reaction can be used to identify the degree of hazard for different reactions (and, to some extent, the length of time required in the reactor). As given in Table 6.3, “highly exothermic or highly endothermic reactions” have heats of reaction i 150 MJ/kmol. Here are some examples.

6.2 General Guideline 197
xExtremely exothermic: direct oxidation of hydrocarbons with air; chlorinations, polymerizations of polyethylene without diluent: i 3 MJ/kg ( or i 150 MJ/kmol: examples: combustion –900 MJ/ kmol; hydrogenation of nitroaromatics, –560 MJ/kmol; nitrodecomposition, – 400 MJ/kmol; diazo-decomposition, –140 MJ/ kmol).
xStrongly exothermic: nitrations, polymerization of propylene, styrene butadiene. 1.2–3 MJ/kg (nitration, –130 MJ/kmol; amination –120 MJ/kmol; sulfonation or neutralization with sulfuric acid, –105 MJ/kmol; epoxidation, –96 MJ/kmol; diazotization).
xModerately exothermic: condensations or polymerization reactions of species with molar mass 60–200 = 0.6–1.2 MJ/kg.
More information is given in Tables 6.3 and 6.1.
6.2.2
Specific Guidelines for Gas Phase, Liquid Phase and Gas–Liquid Reactions
For gas reactions, usually use plug flow tubular reactors, PFTR, static mixers or fluidized beds.
For gaseous, fixed-bed catalytic reactions, use L/Dp i 100 and Dp/D I 0.10 to ensure good gas distribution and negligible back mixing. L = length, D = tube diameter, and Dp = diameter of the solid particle or catalyst.
For liquid phase reactions, usually use continuous stirred tank reactors, CSTR, unless the pressure is so high that the rotating shaft of the agitator cannot be sealed. For such high pressures consider the use of static mixers. Most liquid phase reactions are exothermic.
Prefer operating temperatures below the boiling temperature or dilute the liquid with a safe solvent (H).
For gas–liquid reactions, usually use aerated CSTR or bubble columns.
For GL reactions, whether the reaction is controlled by gas phase mass transfer, rate of mass transfer through the liquid film resistance at the surface or the reaction rate affects the configuration we select for the reactor. Two parameters that show where the reaction occurs are the Hatta number, Ha, and the dimensionless bulk/film volume ratio (ratio of the total liquid volume to the film volume), d+.
The Hatta number is the ratio of the reaction in the liquid surface/mass transfer into the bulk phase; or a modified Thiele modulus for GL systems to correct the mass transfer for chemical reaction. Because the ratio involves the reaction rate, the actual form of the Hatta number depends on the reaction kinetics. For first order reactions,

198 6 Reactors
Ha2 = kA cA d/kL (cA – 0)
where kA = reaction rate/unit surface area,
d = liquid film thickness causing resistance to mass transfer at the surface, which, for the film theory, = D/kL
D = diffusivity of the gaseous reactant species in the liquid. kL = mass transfer coefficient in the liquid phase
or
Ha =( kA D)0.5/kL
More complex expressions apply for reactions of order other than first. The ratio of the total liquid phase to the film volume
d+ = bulk/film volume ratio = (1 – e)/a d = (1 – e) kL/a D
Values of d+ are given for different types of reactors.
d+ = 1 for equipment with a very dry foam so that all the liquid in the reactor is the foam film;
10 I d+ I 200 for thin film reactors (Section 6.19) 10 I d+ I 100 for packed columns (Section 6.16).
d+ is i 100 for mechanically stirred reactions (STR and CSTR, Sections 6.27 and 6.28) and for bubble columns (Section 6.13).
Values of d+ are given for different GL contactors in Table 1.2.
The implications of the Hatta number, and thus the d+ characteristic of the reactor that should be selected, are summarized in Table 6.7.
Table 6.7 Use of the Hatta number to guide in the selection of GL reactors.
Zone |
Ha |
Speed of |
Location |
Controlled |
An increase |
Equipment |
|
|
|
|
reaction |
of |
by |
in tempera- |
|
|
|
|
|
|
reaction |
|
ture causes |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
should give |
d+ values |
Example: |
|
|
|
|
|
|
|
|
|
1 |
II 0.3 |
very slow |
bulk |
reaction |
increase in |
large |
large |
bubble |
|
|
|
|
kinetics, kA |
conversion |
liquid holdup |
|
column; |
|
|
|
|
|
|
|
|
sparged |
|
|
|
|
|
|
|
|
STR |
|
|
|
|
|
|
|
|
|
2 |
between |
|
mostly |
some mass |
|
both holdup |
|
packed |
|
0.3 to 0.6 |
|
in bulk |
transfer |
|
and area |
|
|
|
|
|
|
effects |
|
|
|
|
|
|
|
|
|
|
|
|
|
3 |
between |
inter- |
mostly |
strong mass |
|
both holdup |
|
trays |
|
0.6 and 3 |
mediate |
in film |
transfer |
|
and area |
|
|
|
|
|
|
effects |
|
|
|
|
|
|
|
|
|
|
|
|
|
4 |
i 3 |
very fast |
surface |
mass |
usually |
large surface small |
spray, |
|
|
|
|
film |
transfer, kL |
negligible |
area |
|
wetted |
|
|
|
|
|
effect |
|
|
wall, |
|
|
|
|
|
|
|
|
trickle bed |
|
|
|
|
|
|
|
|
|

6.2 General Guideline 199
6.2.3
Specific Guidelines for Reactions using Catalysts
Catalysts improve the reaction rate and selectivity of reactions. They reduce the energy barrier or activation energy for reactions; uncatalyzed reactions have activation energies in the range 100 to 500 MJ/kmol whereas with most catalysts the activation energies are in the range 50 to100. The temperature effects on the reaction rate overwhelm other effects when activation energy i 30–50 MJ/kmol. Catalysts can be heterogeneous, homogeneous or enzymes. Heterogeneous, solid catalysts usually consist of (i) active species from Groups VIb, VII, VIII and Ib such as elements, oxides, sulfides and carbides, (ii) supports with structural stability and high surface area (such as alumina, carbon, silica, clays with internal pore density 15 m2/g (for a-alumina support), 2–30 m2/g (for kieselguhr); 200 m2/g (for g-alumina support) and 200–800 m2/g (for silica gel)), and (iii) promoters to enhance the activity and selectivity (such as alkali earth metals and metal oxides). Examples of the active species used for different types of reactions are given in Table 6.8.
A measure of the catalyzed reaction rate is the turnover frequency, TOF, = number of molecules reacting per second per active site. Usual values for commercial heterogeneous solid catalysts are, TOF = 0.1–1 1/s; for heterogeneous metallocene catalysts for polymerization reactions, TOF = up to 30 000 ethylene units/s; and for enzyme catalysts: TOF = 10–10 000 1/s. For catalyzed reactions, the controlling step is often the mass transfer. The density of most solid catalysts, is the density of the support and is 1.4 to 2.5 Mg/m3 of solid (although a nonmetallic resin catalyst has a density of about 0.7). The bulk density of most beds of catalyst, rb, (that accounts for the porosity or fluid voidage, e is in the general range 0.5–0.9 Mg/m3 of reactor volume where rb = (1 – e ) rs.
In commercial operation, the solid catalysts usually lose their effectiveness because of (i) poisons (such as sulfur, chloride), (ii) sintering, (iii) fouling by carbon and coke, and (iv) loss of the active species via volatilization. Furthermore, the life of the catalyst depends partly on the thermal stability of the support/carrier. Poisoning is minimized by the removal of poisons from the feed stream by upstream guard units. Sintering is minimized by operating at lower temperatures. Regeneration can be used to periodically remove the coke and carbon; active species may be added.
Enzyme catalysts deactivate especially for temperatures i 50 hC and inadequate control of such conditions as pH.
Table 6.8 also gives example information about the catalyst life, causes of loss in activity and regeneration options.
Table 6.9 indicates how the characteristics of the catalyst affect the type of reactor. Prefer monolithic configuration for intensification (H) with surface area/ volume usually 1.5–4 times greater than traditional pellets. The monolithic configuration is excellent for mass transfer controlled reactions.

Table 6.8 Types of reactions and suggested catalysts with illustrative catalyst life and regeneration information.
Type of reaction |
Example |
Products/ |
Temperature, hC |
Catalyst life |
Cause of decay |
Max. temp., hC |
Comments about |
|
catalysts |
reactants |
|
|
|
|
regeneration |
|
|
|
|
|
|
|
|
Acetylation |
Pd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acetoxidation |
homogeneous |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Addition |
metallic salt in |
|
|
|
|
|
|
|
solution |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aldolization |
homogeneous |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CaO, Mg |
|
|
|
|
|
|
|
aluminate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Alkylation |
molecular sieves |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
zeolite |
ethyl benzene |
420–450 |
i 3 years |
|
|
regeneration (for |
|
|
|
|
|
|
|
24 h) of cat. every |
|
|
|
|
|
|
|
15–30 days |
|
|
|
|
|
|
|
|
|
AlCl3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
sulfuric, HF acid |
|
|
|
|
|
|
oxides of V, Mn, Fe, Cu, Mo, W
Amination |
mixed oxides |
|
|
|
|
via ammonolysis |
|
|
|
|
|
|
|
|
|
|
|
|
silica alumina |
aniline ex |
385 |
1–2 months |
regenerate for |
|
|
phenol, ammonia |
|
|
20 h |
|
|
|
|
|
|
Reactors 6 200
