
Molecular and Cellular Signaling - Martin Beckerman
.pdf
12.1 GPCRs Classification Criteria |
277 |
TABLE 12.1. Three main classes of G protein-coupled receptors: Receptor subtypes are listed to the right of the ligand. G protein-coupled receptors are classified into families according to their structure and the presence or absence in them of a number of highly conserved sequences.
Class A: Rhodopsin family |
Class B: Secretin family |
Class C: Glutamate family |
Adrenergic a1, a2, b1, b2 |
Calcitonin CTR |
Calcium CaR |
Angiotensin AT1A, AT1B, AT2 |
Glucagon GR |
GABA GABABR1 and |
|
|
GABABR2 |
Dopamine D1 to D5 |
Latrotoxin CL1, CL2, CL3 |
Glutamate mGluR1 to |
|
|
mGluR8 |
Histamine H1, H2 Melanocortin MC1, . . . , MC5 Melatonin ML1A, ML1B Muscarinic acetylcholine
m1, . . . , m5
Neurokinen NK1, NK2, NK3 Opioid delta (d), kappa (k),
mu (m)
Prostanoid EP1, . . . , EP4, DP, FP, IP, TP
Purine A1, A2A, A2B, A3, P2Y1, . . . , P2Y6
Serotonin 5-HT receptor subtypes
Somatostatin SST1, . . . , SST5 Vasopressin V1A, V1B, V2
PTH/PTHrP PTH/PTHrPR
Secretin SCTR
VIP/PACAP VIP1, VIP2
list of smaller groups with a few members each. Some of the more prominent G protein-coupled receptors belonging to the three major families are listed Table 12.1. Most of these receptors form small subfamilies. Each receptor has several subtypes and each subtype may be expressed in one of a number of alternative spliced forms.
Class A contains most of the known GPCRs. It is frequently referred to as the rhodopsin family, named for the rhodopsin molecule responsible for transducing photons in rod cells of the retina. Receptors for a diverse spectrum of ligands including most of the amide and peptide hormones and neuromodulators belong to this family. Class A receptors bind a number of glycoprotein hormones such as LH, TSH, and MSH using a large extracellular domain. The glycoprotein-binding receptors differ from the GPCRs for the smaller ligands. The latter receptors rely on extracellular loops or utilize a binding pocket formed by the H3 to H6 helices.
Class B GPCRs, the secretin family, include calcitonin, a 32-amino acid peptide hormone released by “C” cells in the thyroid gland that regulate calcium concentrations in the blood. Secretin, a hormone released by “S” cells in the small intestine, stimulates the pancreas to secrete fluids that regulate acidity during digestion. Parathyroid hormone (PTH) and parathyroid hormone related protein (PTHrP) regulate bone and mineral
278 12. Signaling in the Endocrine and Nervous Systems Through GPCRs
homeostasis. Many ligands for Class B GPCRs such as glucagon, secretin, and VIP/PACAP (vasoactive intestinal peptide/pituitary adenylate cyclaseactivating polypeptide) are fairly large. Receptors in this family utilize an extracellular domain that is far larger than the one depicted in Figure 12.3, plus nearby extracellular loops, for ligand binding.
Class C receptors include the metabotropic glutamate and GABAB receptors mentioned earlier. This collection of GPCRs is also known as the glutamate family. Receptors that signal the presence of extracellular calcium (CaRs) also belong to this group as do a number of mammalian pheromone receptors. An extensive extracellular domain and a large intracellular domain characterize this group. The extracellular domain of these proteins contains two lobes that clamp down on the ligand. This extracellular domain is homologous to bacterial periplasmic binding proteins (PBPs). These are proteins found in the periplasmic space between the outer and inner membranes in gram-negative bacteria. It is speculated that at some time in the past genes encoding PBPs fused with those specifying integral membrane proteins resulting in the formation of Class C GPCRs.
12.2Study of Rhodopsin GPCR with Cryoelectron Microscopy and X-Ray Crystallography
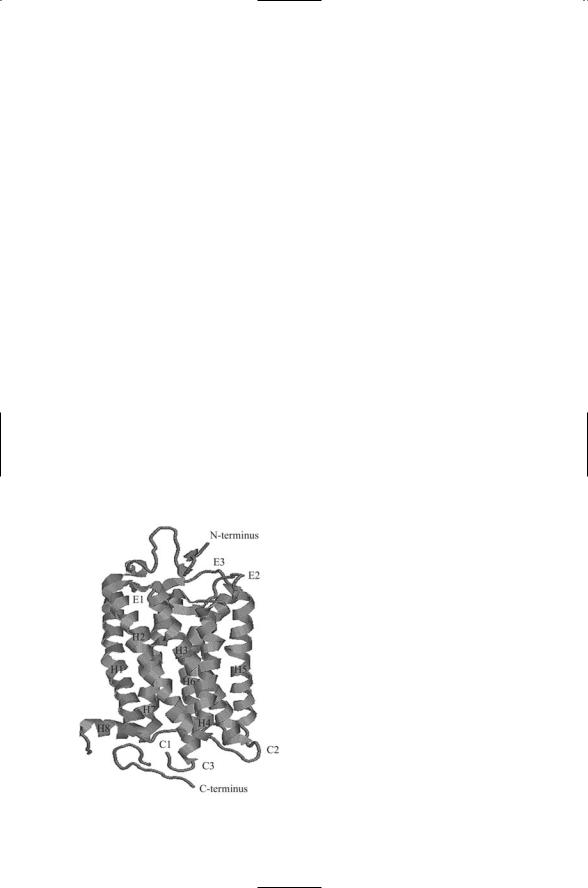
Cryoelectron microscopy and X-ray crystallography studies of rhodopsin have provided detailed information on how GPCR helix bundles are organized and how the GPCR activates its heterotrimeric G proteins. As shown in Figure 12.2 the seven alpha helices in rhodopsin are arranged sequen-
FIGURE 12.2. Structure of rhodopsin determined by means of X-ray crystallography: The rhodopsin molecule contains seven plasma membrane spanning helices (H1 through H7) plus a small C-terminus loop H8. The helices are connected to each other by extracellular loops E1–E3 and cytosolic loops C1–C3. The figure was prepared using the Protein Explorer and atomic coordinates deposited in the Brookhaven PDB under accession code 1F88.

12.3 Subunits of Heterotrimeric G Proteins |
279 |
tially in a clockwise manner when viewed from the intracellular side. Helices 1, 2, 3, and 6 are tilted. That is, their axes are inclined by about 25 degrees relative to an axis drawn perpendicular to the surface. Helices 4 and 7 are nearly perpendicular to the membrane bilayer. Helix 6 is bent; one part is nearly perpendicular to the plane of the membrane and the other part is inclined by about 25 degrees (helix 5 is also bent, but to a lesser extent). In the electron density plot of rhodopsin, the four tilted helices form a central band. Helix 4 lies on one side of the band and helices 6 and
7 lie on the other side.
The rhodopsin molecule operates in a switchlike manner to activate the G protein. Two of the helices, H3 and H6, project into the cytoplasm further than the others. Ligand binding, or photoisomerization in the case of rhodopsin, stabilizes the receptor in an alternative conformation in which there is a shift in the relative positions of helices H3 and H6. When activated by ligand binding the GPCR acts as a guanine nuclcotide exchange factor, or GEF, for its G protein. In its active shifted conformation, the rhodopsin GPCR is able to catalyze the release of GDP from the alpha subunit of the G protein leading to its binding GTP. The GDP-GTP exchange triggers the dissociation of Gabg into Ga and Gbg subunits and the subunits’ migration towards their effectors. The switch is reset by the dissociation of the ligand from the GPCR.
12.3 Subunits of Heterotrimeric G Proteins
As the name implies, heterotrimeric G proteins are assembled from three distinct subunits. These subunits are designated as Ga, Gb, and Gg. There are 20 different Ga subunits, 6 known Gb subunits and 12 distinct Gg subunits. The Ga subunits function as GTPases. Like the Ras GTPase discussed in the last chapter the signaling activity of a Ga subunit is turned off when it is GDP-bound and switched on when it is GTP-bound. The GPCR provides the activation signal and also serves as the GEF, catalyzing the dissociation of bound GDP from Ga. A family of proteins called regulators of G protein signaling (RGS proteins) function as GAPs for the Ga subunits. They catalyze the hydrolysis of GTP by the Ga subunits, thereby rapidly switching off Ga signaling.
There are four kinds of G protein alpha subunits. For most GPCRs one type of GPCR couples to and activates only one of the four kinds of alpha subunits. However, in some cases, the coupling is richer and the GPCR can switch from one kind of subunit to another. Several portions of the
GPCR contribute to the G protein-specific binding. Regions at the ends of helices H3, H5, and H6, loops C2 and especially C3, and the short helix (H8) located in the C-terminal region just after helix H7 are all involved in coupling and activating the various members of the heterotrimeric G protein family.

280 12. Signaling in the Endocrine and Nervous Systems Through GPCRs
Once activated the Ga and Gbg subunits are free to diffuse laterally along the cytoplasmic surface of the plasma membrane, and bind nearby signaling targets, or effectors. The cycle of activation and signaling is completed with hydrolysis and reassociation of the Ga and Gbg subunits. Upon binding Ga, the Gbg induces substantial conformational changes and increases the affinity of Ga for GDP. When bound to Ga, the Gbg subunit cannot bind its effectors and signal because the binding sites on Gbg for its effectors overlap that for Ga.
12.4 The Four Families of Ga Subunits
Four families of Ga subunits are presented in Table 12.2. As shown in the table, Gs subunits bind to and stimulate adenylyl cyclases, while Gi subunits inhibit these effectors. Gq subunits stimulate phospholipase C, and the effectors of G12 subunits are as yet unidentified. Each Ga family contains several variants. Some variants and groups of variants are found in certain tissues while other are more broadly distributed. For example, G0 subunits are brain-specific and the Gi family is sometimes designated as Gi/0 to reflect the inclusion of G0 subunits in this family. Figure 12.3 illustrates how the Gs alpha subunit binds to the C1 and C2 domains of adenylyl cyclase.
Gbg subunits target many of the same second messenger generators as the Ga subunits. Like Ga subunits, some Gbg subunits stimulate adenylyl
TABLE 12.2. Mammalian Ga subunits: G proteins consist of a Ga subunit that is a GTPase, and Gb and Gg subunits that remain attached and function as a unified Gbg subunit. The four different types of G protein alpha subunits are presented in column 1. Different subtypes are listed in column 2 and their effectors (substrates) are given in column 3. Tissue-specific G proteins are noted in column 4. The actions of the subunits on their effectors are given by the plus and minus signs. Plus signs denote stimulation and minus signs indicate inhibition. Abbreviations: adenylyl cyclase (AC), cyclic guanosine monophosphate (cGMP), phosphodiesterase (PDE), phospholipase A2 (PLA2), and phospholipase C (PLC).
Family |
Gene variants |
Effectors |
Association |
Gs |
as(S), as(L) |
+AC |
|
|
aolf |
+AC |
Olfactory |
Gi |
ai1, ai2, ai3 |
-AC |
|
|
a0a, a0b |
+PLC, +PLA2 |
Brain |
|
at1, at2 |
+cGMP, PDE |
Retina |
|
ag |
+PLC |
Gustatory |
|
az |
-AC |
|
Gq |
aq, a11, a14, a15, a16 |
+PLC |
|
G12 |
a12, a13 |
|
|

12.5 Adenylyl Cyclases and Phosphodiesterases |
281 |
cyclases while others inhibit them. Still other Gbg subunits stimulate phosopholipase C. Thus, Ga and Gbg subunits jointly determine the overall action of a G protein on second messenger generators.
12.5Adenylyl Cyclases and Phosphodiesterases Key to Second Messenger Signaling
There are nine types of adenylyl cyclases. These isoforms are designated as types I to IX. All of these isoforms can be stimulated by Gs subunits. However, such is not the case for the Gi subunits. Adenylyl cyclase isoforms I, V, and VI are the only ones inhibited by Gi subunits; the others are not affected. The isoforms also differ in their responses to Gbg subunits, and to the intracellular Ca2+ concentration [Ca2+]i. These response properties are summarized in Table 12.3. As can be seen in Table 12.3, five of the AC isoforms can be regulated by calcium. Thus, the two major second messenger systems are tightly coupled to one another. This coupling can produce a variety of effects including synchronized oscillations in the intracellular cAMP and Ca2+ concentrations.
The magnitude and duration of cyclic nucleotide second messenger signaling is regulated by nucleotide phosphodiesterases (PDEs). Recall that cAMP has a phosphate group attached to both the 3¢ carbon and 5¢ carbon of the sugar ribose. PDEs are enzymes that catalyze the hydrolytic cleavage of 3¢ phosphodiester bonds in cAMP resulting in its degradation to inert 5¢AMP. They also carry out the same operation in cGMP to yield inert
TABLE 12.3. Response properties of adenylyl cyclases to different regulators: The nine isoforms of adenylyl cyclase (AC) are listed in column 1. Columns 2–5 show how the four different kinds of regulators influence them. Stimulatory effects are denoted by plus (+) signs and inhibitory influences by minus (-) signs.
AC Isoform |
Gs |
Gi |
Gbg |
Ca2+ |
I |
+ |
- |
- |
+ |
II |
+ |
|
+ |
|
III |
+ |
|
|
- |
IV |
+ |
|
+ |
|
V |
+ |
- |
|
- |
VI |
+ |
- |
|
- |
VII |
+ |
|
+ |
|
VIII |
+ |
|
|
+ |
IX |
+ |
|
|
|
|
|
|
|
|

282 12. Signaling in the Endocrine and Nervous Systems Through GPCRs
FIGURE 12.3. Complex of the Gs alpha subunit bound to the C1 catalytic domain of adenylyl cyclase V and the C2 domain of adenylyl cyclase II: Shown is a view of the complex looking up from inside the cell towards the plasma membrane. The main features of the interface between the G alpha subunit and the adenylyl cyclase catalytic units consist of Sw I and Sw II of the Ras-like domain of G alpha that protrude into a cleft formed by the alpha 1 to 3 helices of the C2 domain. The figure was prepared using Protein Explorer with atomic coordinates deposited in the Brookhaven Protein Data Bank under accession code 1AZS.
5¢GMP, thereby terminating second messenger signaling. They modulate these signals with regard to their amplitude and duration, and through rapid degradation restrict their spread to other compartments in the cell.
12.6Desensitization Strategy of G Proteins to Maintain Responsiveness to Environment
In order for a cell to maintain its responsiveness to future changes in environmental conditions as relayed through GPCR signaling, it must terminate current GPCR responsiveness to persistent ligands in a timely fashion. The process whereby a GPCR, or any other signaling entity, loses responsiveness to binding by its ligand is called desensitization. The turning off of the GPCR is accomplished in a sequential manner by G protein-coupled receptor kinases (GRKs) and arrestins.
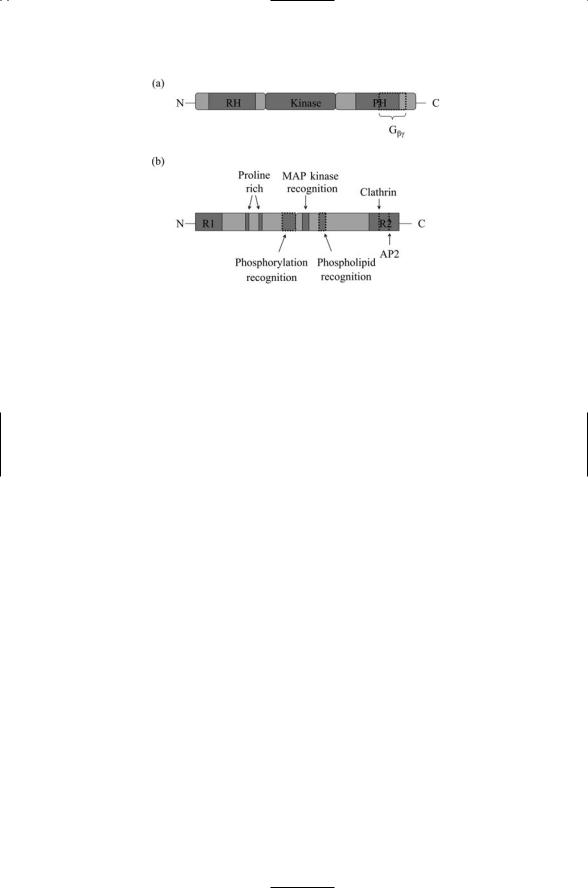
The domain structure of GRK2 and b-arrestin 2 are presented in Figure
12.4. Recall from Chapter 6 and Table 6.1 that G protein-coupled receptor kinases are members of the AGC family of serine/threonine kinases. As shown in Figure 12.4a, the GRK2 member of this family has a central kinase domain plus two flanking regulatory domains. The N-terminal domain contains an RGS homology (RH) domain that characterizes the family. In addi-
12.6 Desensitization Strategy of G Proteins to Maintain Responsiveness |
283 |
FIGURE 12.4. GRK2 and b-arrestin 2 regulators of GPCR signaling: (a) G proteincoupled receptor kinase 2 (GRK2) consists of three domains. The RGS homology (RH) and PH domains occupy the major portions of the N-terminal and C- terminal domains, respectively. (b) b-arrestin 2 has two domains, an N-terminal domain and a C-terminal domain. A pair of regulatory segments, R1 and R2, resides at the ends of the N- and C-terminals. The R2 domain contains clathrinand AP2-binding motifs. Other binding motifs are distributed through the N- and C- terminal domains.
tion to these two domains, GRK2 (but not all GRKs) contains a C-terminal Plectstrin homology (PH) domain. PH domains bind phospholipids and also proteins. The GRK2 C-terminal half of this domain together with several residues lying just outside this domain binds Gbg. The three-dimensional crystal structure of a Gbg subunit bound to GRK2 is presented in Figure 12.5.
The structure of b-arrestin 2 is presented in Figure 12.4b. b-arrestins are adapter proteins devoid of any catalytic ability. In place of a catalytic domain, members of this family possess a number of protein-protein binding domains and motifs that enable them to function as adapters and scaffolds. The b- arrestins contain an N-terminal domain and a C-terminal domain. Their proline-rich region in the N-terminal domain and MAP kinase recognition domain in the C-terminal region provide the protein with the capability of signaling to Src and MAP kinases. In addition, b-arrestins have a phosphorylation recognition domain that mediates binding to the cytoplasmic tail of the GPCR following its phosphorylation by the GRKs. They also have clathrin and AP2 motifs in the C-terminal region that mediate their interactions with the molecular machinery responsible for endocytosis.
The recruitment and subsequent termination of signaling by the arrestins is mediated by a negative feedback loop that automatically prevents excessively sustained signaling. Ligand binding activates the GPCRs, leading to activation of G proteins. The Gbg subunits bind (Figure 12.5) and activate

284 12. Signaling in the Endocrine and Nervous Systems Through GPCRs
FIGURE 12.5. G protein-coupled receptor kinase (GRK) bound to the Gbg subunit of a heterotrimeric G protein: The structure of the complex determined by means of X-ray crystallography. The three main domains of GRK—the N terminal RGS homology (RH) domain, kinase domain and C-terminal PH domain—are labeled in the figure along with the Gb (dark gray) and Gg subunits (light gray) of the G protein. The figure was prepared using Protein Explorer with atomic coordinates deposited in the PDB under accession number 1OMW.
the G protein-coupled receptor kinase, which phosphorylates the receptor. In response the b-arrestins are recruited to and bind the phosphorylated residues using their phosphorylation domain. The GPCR is then no longer able to active the G proteins and signaling ceases through this route. Thus, signaling through this receptor is automatically shut down after a certain time has elapsed.
Densensitization of the GPCRs is also promoted by second messengeractivated protein kinases. Protein kinase A activation mediated by Gs subunits and protein kinase C by Gq subunits both contribute to receptor desensitization. The third intracellular loop and the cytoplasmic tail of the
GPCRs are primary sites of interaction with cytoplasmic proteins. The presence of consensus phosphorylation sites in these regions enables phosphorylation by these kinases. The phosphorylation by PKA of a GPCR to turn off signaling establishes a negative feedback loop that acts through Gs subunits to stimulate cAMP production resulting in PKA activation that shuts off further action by Gs subunits.
12.7GPCRs Are Internalized, and Then Recycled or Degraded
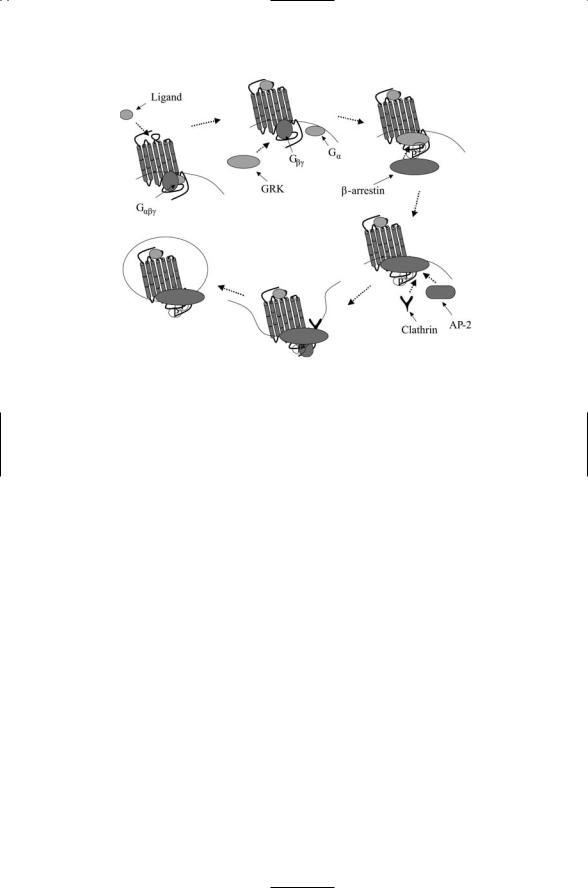
The GPCRs go through a life cycle of activation, signaling, deactivation, and internalization resulting in either degradation or recycling. As illustrated in Figure 12.6, the first steps in this process are the same as discussed in the previous section involving Gbg subunits, namely, GRK2 binding and
12.8 Hormone-Sending and Receiving Glands |
285 |
FIGURE 12.6. Receptor internalization: Activation and G protein signaling is followed by desensitization and signaling that is independent of G proteins. Ligand binding to a GPCR activates the G protein and signaling via second messengers. The Gbg subunit recruits a G protein-coupled receptor kinase (GRK) to the GPCR, which is then phosphorylated by the GRK. This action creates a docking site for b- arrestin, which upon binding to the GPCR blocks further G protein-mediated signaling. Proteins involved in endocytosis such as clathrin and AP-2 attach to the b-arrestin, which now acts as a scaffold for assembly for factors required for vesicle formation and internalization. In the internalization, the ligand-receptor complexes are dissociated and, depending on the type of GPCR, the receptors are either degraded or recycled rapidly or slowly.
phosphorylation, and b-arrestin recruitment. The subsequent recruitment of clathrin and AP-2 enables the packaging of the loaded GPCRs into endocytic vesicles that pinch off from the plasma membrane. The cargo consisting of ligand-bound receptors, b-arrestins, and perhaps additional signaling proteins such as Src are then delivered to endosomes for recycling to the cell surface or for shipment to lysosomes for degradation. This kind of life cycle is not restricted to GPCRs. Receptor tyrosine kinases such as the Trks discussed in the last chapter are also packaged into endodomal vesicles for internalization and recycling.
12.8 Hormone-Sending and Receiving Glands
The hypothalamus, anterior pituitary gland, adrenal gland, and the endocrine pancreas send and receive hormones. While polypeptide hormones

286 12. Signaling in the Endocrine and Nervous Systems Through GPCRs
signal through receptor tyrosine kinases, as was discussed in Chapter 11, other nonlipophilic hormones secreted by cells in the hypothalamus, anterior pituitary, adrenal gland, and endocrine pancreas signal through GPCRs.
Hypothalamus. The hypothalamus controls the release of hormones by the anterior pituitary. The hypothalamus produces two hormones that inhibit hormonal release by the pituitary, and five hormones that stimulate the release of pituitary hormones. One of these five, dopamine, is a catecholamine, a molecule composed of an aromatic (catechol) part, i.e., a benzene ring plus two hydroxyl groups, and an amine part. The other hormones are peptides that vary in length from 3 amino acids (TRH) to 44 amino acid residues (GHRH). These neurohormones all bind to GPCRs on cells of the anterior pituitary.
Anterior pituitary. The anterior pituitary produces hormones that control hormone release elsewhere in the body. Five types of cells secrete anterior pituitary hormones. Somatotrophic cells secrete growth hormone and lactotrophic cells produce prolactin. Growth hormone and prolactin are bound by single chain cytokine receptors, and not by GPCRs. Human growth hormone (hGH) receptor recognition was discussed in Chapter 8. Growth hormones circulate through the body and influence all cell types. Prolactin influences mammary gland development. Corticotrophic cells secrete corticotropin and melanocyte-stimulating hormone (MSH). Corticotropin influences the adrenal gland while MSH targets melanocytes, melanincontaining skin cells. ACTH and MSH are both derived from the precursor molecule, pro-opiomelanocortin (POMC). Another molecule derived from POMC is the neuromodulator beta-endorphin. Gonadotrophic cells secrete follicle-stimulating hormones (FSH) and lutenizing hormones (LH), and thyrotrophic cells produce thyroid-stimulating hormone (TSH). TSH, FSH, and LH are large glycoproteins. The addition of carbohydrate groups to these hormones extends their lifetime by protecting them from degradation. Gonadotropins stimulate a variety of responses in men and women including sperm development, androgen release, estrogen synthesis, and ovulation. These pituitary hormones with the exception of GH and PRL all bind to GPCRs expressed by cells of the above mentioned tissues and organs. The hormone sources and acronyms are summarized in Table 12.4.
Adrenal gland. Chromaffin cells in the adrenal gland produce two cate- cholamines—adrenaline (epinephrin) and noradrenaline (norepinephrin).
These hormones, secreted in response to extreme stress, trigger an increased blood flow and other physiological “fight or flight” reactions. They are derived from tyrosine and bind to adrenoreceptors, adrenergic GPCRs distributed in smooth muscle of the vascular system and gastrointestinal tract,
