
Molecular and Cellular Signaling - Martin Beckerman
.pdf
9.13 Interferon System |
205 |
9.13Interferon System: First Line of Host Defense in Mammals Against Virus Attacks
Interferons are secreted into the bloodstream where they circulate to all cells in the body to alert them that a virus attack is underway. They trigger an organism-wide, or global, response to the virus attack in cells that have not been attacked along with those that have been invaded. Invasion of a virus and the resulting viral replication stage produce dsRNA (doublestranded RNA) molecules. These are present in the cell only when a virus has invaded and started replication. The presence of dsRNA sets off a series of regulatory events. A resident cellular sensor of dsRNA called protein kinase R (PKR) is activated by the dsRNAs. The PKR proteins activate a NF-kB, and IFN regulatory factor (IRF), which binds within the IFN stimulated responsive element (ISRE) to promote transcription of the antiviral proteins including the interferons. In addition, PKR signals other control proteins to halt all protein synthesis (Figure 9.10). This action blocks the virus’ ability to replicate and make proteins that it needs. The overall process operates though a negative feedback loop. The presence of dsRNA is required for action by PKR, and when this quantity is reduced the block on protein synthesis is relieved.
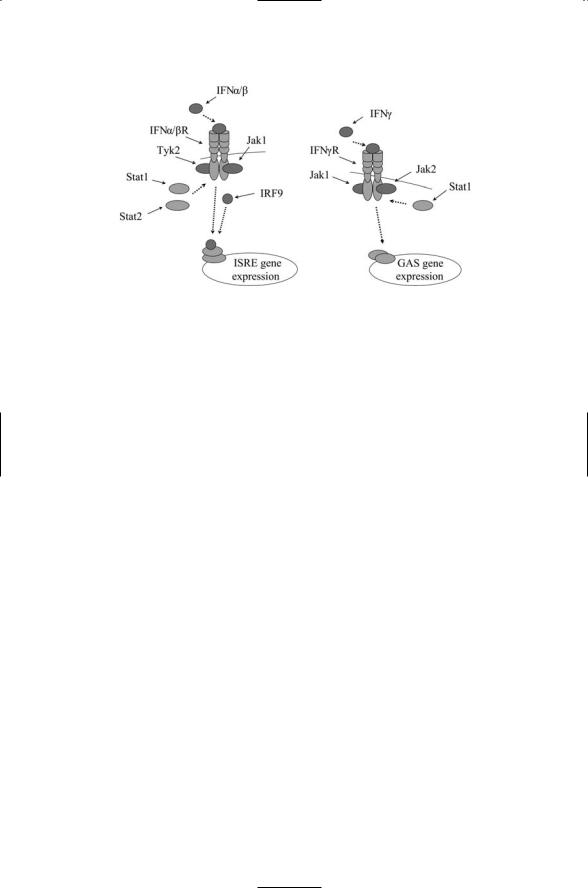
Interferon signal transduction is initiated when a ligand binds to an interferon receptor dimer (Figure 9.11). As is typical of the hematopoietins this event stimulates the recruitment to the plasma membrane and activation of members of the Janus family of protein tyrosine kinases. Phosphorylation stimulates the further recruitment of the STAT proteins, which are phosphorylated by the Jaks. In the case of IFNa/b- mediated signaling, Jak1 and Tyk2 phosphorylate STAT1 and STAT2, which then form heterodimers that further associate with an IRF protein.
FIGURE 9.10. Antiviral activities of PKR: Protein kinase R, a sensor of dsRNAs, stimulates production of interferons by activating NF-kB and IRF (not shown) transcription factors, and halts protein synthesis by activating eIF2a, a negative regulator of protein synthesis.
206 9. Signaling by Cells of the Immune System
FIGURE 9.11. Interferon signaling: Displayed in the figure are the main components of the IFNa/b and IFNg pathways from the plasma membrane to the nucleus. Distinct a and b chains of each receptor jointly bind a ligand. Janus family kinases are recruited and are activated. They create docking sites for the STATs by phosphorylating the receptors. The STATs are recruited and are phosphorylated by the Jaks. STATs form dimers (trimers) and translocate to the nucleus where they bind to interferon-responsive DNA transcriptional sites, the ISREs and interferm-gamma activated sites (GASs).
The trimers translocate to the nucleus where they stimulate transcription of IFNa/b-responsive genes IFNg utilizes a different set of signal transducers. Jak1 and Jak2 are recruited to the plasma membrane and phosphorylate STAT1s, which form homodimers that translocate to the nucleus where they stimulate transcription of IFNg-responsive genes. Cytokines such as the interferons mount a multilayered response. Besides arrest of protein synthesis and cell multiplication, they stimulate the production of enzymes that kill (degrade) the viruses, recruit cells that attack virus-containing cells, and organize other elements of the adaptive immune response.
9.14Chemokines Provide Navigational Cues for Leukocytes
Chemokines are chemoattractants that guide the movement of leukocytes. Some chemokines are inflammatory chemokines while others are lymphoid chemokines. Inflammatory chemokines attract neutrophils, macrophages, and other leukocytes central to the innate immune response. Inflammatory chemokines are secreted by several different kinds of cells including leukocytes and endothelial cells that line the blood vessels. Lymphoid chemokines help regulate leukocyte traffic and cell compartmentalization within lym-

9.15 B and T Cell Receptors Recognize Antigens |
207 |
phoid tissues. Thus, they act to maintain homeostasis among the various populations of lymphocytes. Stromal cells, endothelial cells, and other cells residing in the lymphoid organs produce these signaling molecules.
Chemokines have a positive charge and bind to heparin and heparin sulfate, negatively charged polysaccharides found within the extracellular matrix and on the surface of endothelial cells. The heparin and heparin sulfate molecule act as receptors for the chemokines. The chemokines become immobilized when bound to the polysaccharide-covered substrates and form stable gradients of chemokine concentration. The migratory leukocytes navigate up the gradients to the target sites.
Chemokines are bound at the cell surface by G protein-coupled receptors (GPCRs), seven-pass transmembrane receptors coupled to G proteins. Their method of transducing signals into the cell was examined briefly in the last chapter and will be discussed in detail in Chapter 12. There are four families of chemokine receptors. These families are distinguished from one another by their structure and by their chromosomal locations. The distinguishing structural feature is the presence of four conserved cysteine (C) residues in specific positions. The CXC and CC families are fairly large with several members each, while the two small families, designated as CX3C and C, have a single known member each. The CXC family has an amino acid located between the first and second cysteines; in the CC family the two cysteines are directly adjacent to one another. There is considerable redundancy; within each ligand-receptor family, the chemokines can bind to more than one type of receptor, and the receptors bind to more than one type of chemokine.
Lymphoid chemokines form chemical highways within lymphoid organs that guide the migration of lymphocytes from site to site bringing together lymphocytes that must interact with one another in the adaptive immune response. The B-cell attracting chemokine (BCA-1), and secondary lymphoid chemokine (SLC) are representative lymphoid chemokines. They bind the CXCR5 and CCR7 receptors, respectively. Gradients of BCA-1 chemokines guide the entry of T cells and dendritic cells into lymphoid organs. Similarly, gradients of SLC are crucial for compartmental homing of B cells and T cells, helping to establish distinct territories within the secondary lymphoid organs.
9.15 B and T Cell Receptors Recognize Antigens
The first stage of the lymphocyte immune response is the production by B cells of antibodies, receptors that bind to specific antigens. There are two classes of antigen-recognizing receptors, one set expressed on B cells and referred to as B cell receptors (BCRs), and the other set expressed on T cells and referred to as T cell receptors (TCRs). Major histocompatability complexes (MHCs) also recognize antigens, and these may be regarded as

208 9. Signaling by Cells of the Immune System
a third class of antigen-recognizing receptors. All are members of a large family of glycoproteins known as immunoglobulins (Igs) that share a similar structure and fold.
Each B cell expresses on its surface a large number of a single kind of Ig molecule. When a B cell encounters an antigen that it recognizes (i.e., that it binds), it matures and differentiates into a plasma cell that produces many more antibodies of that specific type. These antibodies are no longer membrane-bound receptors but instead are secreted and become free to diffuse and bind to their specific antigen whenever that antigen is exposed on the surface of the bacterium or other pathogen. This binding event not only tags that object for destruction by other leukocytes, but also inactivates viruses and bacterial toxins by impeding their ability to attach to their cellular targets.
At least two distinct signals are needed to elicit cellular responsiveness. Antigen binding to the BCR is the first signaling step. The B cell ingests the antigen-antibody complex, processes the antigen, and presents it bound to an MHC Class II molecule on its cell surface. Activated helper T (Th) cells possessing receptors that recognize that specific antigen convey the second signal. Signals relayed into the Th cell trigger the production and release of cytokines by the Th cell. The cytokines are bound to cytokine receptors on the B cell. This second signal triggers B cell differentiation and proliferation and the release of antibodies from the resulting plasma cells.
T cells target pathogens such as viruses and small bacteria that hide inside other cells. Antigen receptors on TCRs recognize short peptide sequences, typically 8 to 15 residues long, belonging to an antigen that has been processed and bound to MHC molecules expressed on the surface of MHCpresenting cells. The TCR recognition process differs from BCR antigen recognition since the latter targets entire molecules, either denatured or native forms of proteins or cell-bound carbohydrates. Both the BCR and
TCR molecules have distinct antigen-binding and signaling units. Unlike the BCR, TCRs are not later made in quantity and secreted but instead remain membrane-bound. The signaling tail of the T cell receptor associates with a set of chains collectively termed CD3. The CD3 chains, and a pair of disulfide-linked z chains, are immunoglobulin family members that assist the TCR molecule in transducing signals into the cell.
9.16 MHCs Present Antigens on the Cell Surface
Almost all cells in the human body express MHC proteins on their surface.
Between 104 and 106 Class I MHCs are present on the surface of a typical nucleated cell, and similar numbers of Class II MHC proteins are expressed on the surfaces of MHC Class II-expressing cells. Each MHC allele can bind some 1000 to 2000 different self-peptides represented at greater than 10 copies per cell and up to several hundred in some cases. Pathogen proteins are encountered at far smaller numbers. 102 to 104 copies accumulate on

9.17 Antigen-Recognizing Receptors Form Signaling Complexes |
209 |
the cell surface. Pathogen peptides trigger responses even though they are only a small fraction of the total peptide fragment population—from 0.01 to 0.1%. Thus, the MHC must exhibit high sensitivity and selectivity of foreign peptides while maintaining low responsiveness to self-peptides.
Different individuals express different mixes of these molecules. These proteins lie at the core of self versus not-self recognition. The job of an MHC is to present antigens to T cells and to NK cells. Class I MHCs interact with CD8 receptors on cytotoxic T cells and with KIR receptors on NK cells. Class II MHCs interact with CD4 receptors on helper T cells. Class I members bind peptides derived from molecules encountered in the cytosol while Class II members bind peptides from molecules broken down in the endosomes.
What the MHCs of both classes have to do is present the broadest range of peptides possible while simultaneously satisfying the requirement that these peptides be presented in a way that maximally stimulates TCR responses. The minimal size of a peptide fragment needed to discriminate between self and foreign is about 8 or 9 amino acid residues. One way of satisfying the broadness requirement is to have several binding sites along the MCH. However, this would violate the TCR stimulation requirement. To maximally induce a TCR response, there should be a single peptidebinding site with each peptide of a given sequence binding to a given MHC allele in exactly the same way. The solution is to have a single binding pocket and a way to generate a spectrum of different MHCs utilizing a combination of conserved and nonconserved (variable region) residues in the binding pocket.
9.17Antigen-Recognizing Receptors Form Signaling Complexes with Coreceptors
The mixes of proteins expressed on the surfaces of leukocytes are unique markers of the population or subpopulation to which the leukocytes belong. The different ensembles of proteins reflect not only the kind of leukocyte but also characterize the different stages of leukocyte development and activation/inactivation. The proteins that can be so used are given a “cluster of differentiation,” or CD, designation because of their association with specific lineages or stages of differentiation. CD proteins function as receptors, coreceptors, and as cell adhesion molecules.
Transmembrane-signaling proteins that form signaling complexes with the BCRs and TCRs are well represented in the CD listing. The two main classes of T cells are distinguished from one another by the presence of either CD4 or CD8 molecules on their surfaces. CD4 cells such as helper T cells signal other leukocytes to converge on the site of an infection. The CD4 molecula acts in concert with T cell receptors to bind peptides derived from antigens bound to MHC-II molecules. (The TCR binds the peptide, while the CD4 molecule binds MHC-II). The T8 group of T cells, such as

210 9. Signaling by Cells of the Immune System
FIGURE 9.12. T cell receptor (TCR) signal transduction pathways leading to expression of genes activated by AP-1, NF-kB, and NFAT transcription factors working in concert: In response to ligand binding at the TCR, CD45 (a receptor tyrosine phosphatase) and CD4 activate Fyn and Lck. Fyn and Lck phosphorylate the g, d, and e chains of the CD3 complex and the z dimer. After phosphorylation, ZAP-70 containing two SH2 domains is recruited and binds to the phosphatyrosines and is then activated through phosphorylation by Lck. ZAP-70 then interacts with PLC-g, which subsequently cleaves PIP2 to produce IP3 leading to the aforementioned increased concentration of intracellular calcium. DAG activates PKC, which serves as the upstream kinase for activation of NF-kB, while ZAP-70 acts through a RasGEF, Ras, and a MAP kinase cascade leading to activation of AP-1. Intermediate steps involved in activation of NF-kB by PKC have been omitted for simplicity.
cytotoxic T cells, express CD8 proteins on their surfaces. The CD8 proteins act in concert with the T cell receptors to bind peptides derived from antigens bound to MHC-I molecules.
Several transmembrane proteins must associate with one another for efficient signaling into the cell. The upstream signaling starts with ligand binding and association of the TCRs with their CD4 or CD8 coreceptors and with CD3 chains (Figure 9.12). CD3 consists of several separate chains, denoted as d, g, and e. In addition, there is a fourth kind of chain, called z. These chains form homoand heterodimers that associate with the a/b chains of the TCR. The CD3 and z chains possess immunoreceptor tyro- sine-based activation motifs, or ITAMs, in their cytoplasmic regions. These enable the CD3 chains to recruit and bind Fyn and Lck protein tyrosine kinases (protein tyrosine kinases will be discussed in detail in Chapter 11), triggering a series of steps leading to activation of AP-1, NF-kB, and NFATresponsive genes.
Signaling through the TCR and its associated proteins culminates in the transcription of genes-encoding receptors, signal transducers, cell adhesion

9.18 Costimulatory Signals Between APCs and T Cells |
211 |
molecules, and cytokines. One of the most prominent of the cytokines upregulated through TCR signaling is IL-2. Compared to the straightforward signal transduction pathways utilized by cytokines, signaling through the TCR is far more complex. The steps leading to IL-2 production, along with many other gene products, involve activation of the lipid second messenger pathways discussed in the last chapter. Intracellular calcium levels are increased through stimulation of phospholipase C activity leading to a greater IP3 production, which increases the concentration of intracellular calcium. The calcium ions along with calmodulin activate the protein phosphatase, calcineurin, which, in turn, dephosphorylates and thus activates the nuclear factor of activated T cells (NFAT) transcription factors. The NFATs work in cooperation with members of the AP-1 family of transcription factors and the NF-kB transcription factor. These transcription factors stimulate the transcription of genes leading to the differentiation and proliferation of the T cells.
9.18 Costimulatory Signals Between APCs and T Cells
The task of discriminating between self and non-self is a difficult one. Several failsafe mechanisms operate to ensure that inappropriate actions are not taken by B cells and T cells. One of the mechanisms is the requirement that there be two independent activating signals before T and B cells are fully activated. The first signals are conveyed by the BCRs and TCRs in association with their CD4 and CD8 coreceptors. The second set of signals is sent into the cell via costimulatory pathways. The most prominent of the costimulatory pathways are the CD28/B7 system and the CD40/CD40L signaling pathway (Table 9.5).
The first entry in Table 9.5 is the CD40 receptor and its ligand. They are members of the TNF superfamily. Costimulatory signals supplied via
CD40 are an important part of the help supplied by helper T cells. The CD40 ligand is found on CD4+ and CD8+ T cells, while the CD40 receptor is found on APCs such as B cells and dendritic cells. Signaling through
TABLE 9.5. T lymphocyte costimulators: Abbrevia-
tions—Inducible |
costimulator |
(ICOS); programmed |
death-1 (PD-1). |
|
|
Receptor |
Ligand |
Comments |
|
|
|
CD40 |
CD40L |
TNF superfamily |
CD28/B7 |
|
Ig superfamily |
CD28 |
B7-1, B7-2 |
Positive regulator |
CTLA-4 |
B7-1, B7-2 |
Negative regulator |
ICOS |
ICOSL |
Positive regulator |
PD-1 |
B7-L1, B7-L2 |
Negative regulator |
|
|
|

212 9. Signaling by Cells of the Immune System
CD40/CD40L activates resting B cells and primes the dendritic cells to adequately stimulate the T cells.
The remaining receptors and ligands all belong to the CD28/B7 branch of the immunoglobulin (Ig) superfamily. The first of these, CD28, is constitutively expressed on the surface of most T cells. Signaling through CD28 helps determine how the T cells will differentiate. In the absence of the costimulatory signals the T cells undergo anergy, becoming nonresponsive, or tolerant, of antigens presented on the surface of the antigen-presenting cells
(APCs). In this state the T cells are inactive. They do not proliferate nor do they send out cytokines signals. The co-stimulator signals not only prevent anergy but also help determine whether the activated T cell will become a helper T cell or an effector T cell.
CD28 and CTLA-4 both bind to a pair of ligands, B7-1 and B7-2. The CTLA-4 receptor is transiently expressed. It has a higher affinity for the B7 ligands than does CD28, and when it is expressed it shuts down the signaling. A second pair of positive and negative regulators, ICOS and PD-1, also appears in the table.These glycoproteins are not constitutively expressed but are inducible through CD28 signaling and act later during immune responses. The positive regulator, ICOS, helps promote B-T cell interactions while PD-1, like CTLA-4, halts cytokine production and the cell cycle progression.
9.19 Role of Lymphocyte-Signaling Molecules
Lymphocyte-signaling molecules form immunological synapses. In order to carry out the kind of signaling required for an immune response several kinds of molecules must come together at and just below the proximal cell surfaces of the T cell and the APC. Signaling into the leukocyte cell interior is triggered at the plasma membrane by surface contact (cell adhesion), by antigen-signaling through the T cell receptors and coreceptors, and by costimulatory signals. Cell adhesion molecules important for this process include integrins such as LFA-1, and cell adhesion molecules belonging to the Ig superfamily, such as the ICAMs. Cell adhesion molecules play a crucial role in leukocyte motility and will be examined in detail in the next chapter.
The transmission of messages across the plasma membrane and into the cell interior is best thought of as a process. It is carried out in several distinct steps. It takes a certain amount of time and covers a region of space. Depending on the character of the message, the transmission can be fairly straightforward or can require several intermediate steps in order to elicit the appropriate cellular response. In either case signal transduction is not carried out by a single gene product but rather by multiple gene products working in close physical proximity and contact with one another. In the immune response the receptors themselves are composed of a number of polypeptide chains. Some of the chains are involved in recognition, others

9.20 Kinetic Proofreading and Serial Triggering of TCRs |
213 |
with transducing a signal from the extracellular face to the cytoplasmic face. The receptors work together with coreceptors and with cell adhesion molecules to relay information into the cell.
In the immune systems receptors cluster and rearrange themselves. Signals received at the cell surface help shape the clusters; the character of the cluster in turn shapes the signal transduced into the cell interior. The lipid bilayer is not passive but instead plays an active role in these adaptive rearrangements. These procedures are utilized by leukocytes, by nerve cells, and, as was discussed in Chapter 7, in the bacterial chemotactic system.
The proteins required for signaling between T cells and APCs are organized into signaling structures called immunological synapses (ISs). The term “synapse” denotes a junction between cells where information is transmitted. Synapses are highly enriched in signaling molecules that not only transmit signals but also adaptively control the properties of the relayed messages such as their strength. The term “synapse” originates in studies of the transmission of signals between nerve cells. The literal meaning of the term, coined by Sir Charles S. Sherrington in 1897, is “to clasp together.” Synapses between nerve cells will be explored in Chapter 21.
Immunological synapses bring together a host of proteins required to support sustained signaling. Among the components of the synapse are receptors, cytoplasmic kinases, and adapters and anchors that link events at the cell surface to the cytoskeleton. The TCRs and MHCs lie in the middle of immunological synapse. As discussed earlier, the TCRs associate with cytoplasmic CD3 complexes, protein tyrosine kinases such as Lck and Fyn, and costimulatory molecules such as CD28 and CD40. Other proteins belonging to the immunological synapse include ICAM-1 immunoglobulins and LFA-1 intergrins, which bind one another, and members of the CD2 grouping of Ig adhesive molecules, which include CD48 (rat) and CD58 (human). This second group of mainly adhesive proteins surrounds the central portion of the immunological synapse.
9.20 Kinetic Proofreading and Serial Triggering of TCRs
The TCR utilizes kinetic proofreading and serial triggering to discriminate between self and non-self antigens. The TCR is remarkable. It can tell self from non-self antigens even when the latter are buried in a sea of self antigens. At first glance, the TCR assemblage looks unwieldy, if not outright baroque. A whole series of diffusion, phosphorylation, and binding events are required to elicit a proper cellular response to antigen binding. Involved are several cytoplasmic proteins, namely, Lck, Fyn, and ZAP-70, a number of adapters, a cluster of transmembrane chains, some of which, most notably the z-z chains associated with CD3, are subjected to multiple phosphorylations, and finally PLC and the RasGTPase. It turns out that these two aspects of TCR signaling are tied together.

214 9. Signaling by Cells of the Immune System
Completion of the series of phosphorylation steps, including those of the z-z chains, depends on the nature of the peptide gripped by the MHC molecule and bound to the TCR. If the ligand is not a non-self antigen, the activation series will not run to completion before the ligand dissociates from the receptor. The dissociation will abrogate the sequence of diffusion and phosphorylation events before a strong (optimal) signal can be generated. By this means differences in lifetime of the receptor-ligand binding are converted to differences in signaling because of the presence of many intermediate timeand energy-consuming steps.
This process is called kinetic proofreading. This term was first introduced in the 1970s to explain how high levels of fidelity could be achieved in operations such as transcription and translation. In these processes, the error rates are exceedingly low, on the order of 10–4 to 10–9. These low error rates are hard to understand using thermodynamic arguments alone because the energy differences between correct and incorrect steps are miniscule. This situation is comparable to that of the TCR, which can tell self from non-self antigens when the latter are present in as few as 1 part in 10,000.
Kinetic proofreading occurs along with another chain of steps called serial triggering. The term “triggering” in this case refers to the generation of a signal by the TCR at the end of the series of internal steps. In serial triggering, a single peptide-bound to an MHC molecule (pMHC) is able to bind to several TCRs, one after the other in a serial fashion. Once the series of diffusions and phosphorylation is completed the TCR is internalized and the pMHC is free to engage another TCR. By this means the effect of a small number of non-self peptides is amplified. Serial engagement favors short lifetimes so that many receptors can be engaged, while kinetic proofreading favors longer ones to increase the fidelity.The result is a balance between the two processes and a dissociation rate optimized for TCR signaling.
The formation of an immunological synapse permits optimal signaling through the TCR. As noted above, in an immunological synapse, a ring of adhesive molecules called the peripheral supramolecular activation cluster
(pSMAC) surrounds a central ring of TCRs and associated signaling proteins, collectively referred to as the central supramolecular activation cluster
(cSMAC). This clustering together of TCRs into a cSMAC accomplishes two things. It promotes serial triggering, and it helps to rapidly turn off signaling through receptor internalization once the signaling has reached its appropriate level.
General References
Benjamini E, Coico R, and Sunshine G [2000]. Immunology (4th edition) New York: John Wiley and Sons.
Janeway CA, Jr., Travers P, Walport M, and Capra JD [1999]. Immuno Biology (4th edition) London: Current Biology Publications.
