
Garrett R.H., Grisham C.M. - Biochemistry (1999)(2nd ed.)(en)
.pdf
20.6 ● Isocitrate Dehydrogenase—The First Oxidation in the Cycle |
651 |
20.6 ● Isocitrate Dehydrogenase—The First
Oxidation in the Cycle
In the next step of the TCA cycle, isocitrate is oxidatively decarboxylated to yield -ketoglutarate, with concomitant reduction of NAD to NADH in the isocitrate dehydrogenase reaction (Figure 20.10). The reaction has a net G° of 8.4 kJ/mol, and it is sufficiently exergonic to pull the aconitase reaction forward. This two-step reaction involves (1) oxidation of the C-2 alcohol of isocitrate to form oxalosuccinate, followed by (2) a -decarboxylation reaction that expels the central carboxyl group as CO2, leaving the product -ketoglu- tarate. Oxalosuccinate, the -keto acid produced by the initial dehydrogenation reaction, is unstable and thus is readily decarboxylated.
Isocitrate Dehydrogenase Links the TCA
Cycle and Electron Transport
Isocitrate dehydrogenase provides the first connection between the TCA cycle and the electron transport pathway and oxidative phosphorylation, via its production of NADH. As a connecting point between two metabolic pathways, isocitrate dehydrogenase is a regulated reaction. NADH and ATP are allosteric inhibitors, whereas ADP acts as an allosteric activator, lowering the Km for isocitrate by a factor of 10. The enzyme is virtually inactive in the absence of ADP. Also, the product, -ketoglutarate, is a crucial -keto acid for aminotransferase reactions (see Chapters 14 and 27), connecting the TCA cycle (that is, carbon metabolism) with nitrogen metabolism.
(a) |
H2C |
|
|
|
|
|
COO– |
||
|
|
|
|
|
|||||
H |
|
|
C |
|
|
|
COO– |
||
|
|
|
|
|
|||||
H |
|
|
|
|
|
|
|
|
COO– |
|
|
C |
|||||||
|
|
|
|
||||||
OH
NAD+
Isocitrate dehydrogenase
NADH 
+ H+
|
H2C |
|
|
COO– |
|
|
CO2 |
|
|
|
|||
|
|
|
|
|
|||||||||
|
|
|
|
|
|
O |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
H2C |
|
COO– |
||
|
|
|
|
|
|
|
|
|
|
||||
H |
|
C |
|
|
C |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
O– |
|
|
|
H2C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
H+ |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
C |
|
|
|
|
C |
COO– |
|
|
|
COO– |
||||||
|
|
|
|
|
O |
||||||||
O |
|
|
|
||||||||||
Oxalosuccinate |
|
|
|
α -Ketoglutarate |
|||||||||
(b)
● (a) The isocitrate dehydrogenase reaction. (b) The active site of isocitrate dehydrogenase. Isocitrate is shown in green, NADP is shown in gold, with Ca2 in red.

652 Chapter 20 ● The Tricarboxylic Acid Cycle
20.7 ● -Ketoglutarate Dehydrogenase—
A Second Decarboxylation
A second oxidative decarboxylation occurs in the -ketoglutarate dehydrogenase reaction (Figure 20.11). Like the pyruvate dehydrogenase complex,-ketoglutarate dehydrogenase is a multienzyme complex—consisting of
-ketoglutarate dehydrogenase, dihydrolipoyl transsuccinylase, and dihydrolipoyl dehydrogenase—that employs five different coenzymes (Table 20.2). The dihydrolipoyl dehydrogenase in this reaction is identical to that in the pyruvate dehydrogenase reaction. The mechanism is analogous to that of pyruvate dehydrogenase, and the free energy changes for these reactions are 29 to 33.5 kJ/mol. As with the pyruvate dehydrogenase reaction, this reaction produces NADH and a thioester product—in this case, succinyl-CoA. Succinyl-CoA and NADH products are energy-rich species that are important sources of metabolic energy in subsequent cellular processes.
20.8 ● Succinyl-CoA Synthetase—A Substrate-
Level Phosphorylation
The NADH produced in the foregoing steps can be routed through the electron transport pathway to make high-energy phosphates via oxidative phosphorylation. However, succinyl-CoA is itself a high-energy intermediate and is utilized in the next step of the TCA cycle to drive the phosphorylation of GDP to GTP (in mammals) or ADP to ATP (in plants and bacteria). The reaction (Figure 20.12) is catalyzed by succinyl-CoA synthetase, sometimes called succinate thiokinase. The free energies of hydrolysis of succinyl-CoA and GTP or ATP are similar, and the net reaction has a G° of 3.3 kJ/mol. Succinyl-CoA synthetase provides another example of a substrate-level phosphorylation (Chapter 19), in which a substrate, rather than an electron transport chain or proton gradient, provides the energy for phosphorylation. It is the only such reaction in the TCA cycle. The GTP produced by mammals in this reaction can exchange its terminal phosphoryl group with ADP via the nucleoside diphosphate kinase reaction:
Nucleoside diphosphate
kinase
3888888888888888888884
GTP ADP 88888888888888888888 ATP GDP
|
|
|
|
|
|
|
|
NADH |
|
|
|
|
|
|
|
|
|
|
|
|
NAD+ |
CoA |
+ H+ |
CO2 |
|
|
|
|
|
H2C |
COO– |
H2C |
|
COO– |
||||||||||
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H2C |
|
|
|
|
|
|
|
|
H2C |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
COO– |
|
|
|
α -Ketoglutarate |
|
|
C |
|
SCoA |
|||
|
|
|
|
|
||||||||||
O |
|
|
|
|
dehydrogenase |
|
|
O |
|
|
||||
|
|
|
|
|
|
|
|
|
|
|||||
α -Ketoglutarate |
|
|
|
|
Succinyl-CoA |
|||||||||
FIGURE 20.11 ● The -ketoglutarate dehydrogenase reaction.



20.11 ● Malate Dehydrogenase—Completing the Cycle |
655 |
|
Carbonium ion mechanism |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
COO– |
|
|
|
|
|
|
COO– |
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
HO– |
|
|
|
|
COO– |
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
+C |
|
|
|
|
H |
|
|
|
HO |
|
|
|
|
|
|||||
|
|
|
|
C |
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
H |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
C |
|
|
|
H |
|
H |
|
C |
|
|
H |
|
|
|
|
|
CH2 |
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
COO– |
|
|
B |
|
|
|
|
COO– |
|
|
|
|
COO– |
||||||||||||||
|
|
Fumarate |
|
|
E |
|
Carbonium ion |
|
|
L-Malate |
|
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carbanion mechanism |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COO– |
|
|
|
|
|
|
|
|
||||||||
HO– |
|
COO– |
|
|
|
|
|
|
|
|
|
COO– |
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
C |
|
H |
|
|
|
|
HO |
|
C |
|
|
|
|
H |
|
|
|
HO |
|
C |
|
H |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
– |
|
|
|
|
|
|
|
|
||||||
|
H |
|
|
C |
|
|
|
|
|
|
H |
|
C |
|
|
|
|
|
CH2 |
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|||
|
|
|
|
COO– |
|
|
B |
|
|
|
COO– B |
|
|
|
|
COO– |
|||||||||||||||
|
Fumarate |
|
|
|
|
E |
|
Carbanion |
E |
L-Malate |
|
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FIGURE 20.18 ● Two possible mechanisms for the fumarase reaction.
and that trans-addition of OH and OOH occurs across the double bond of cis-aconitate. Though the exact mechanism is uncertain, it may involve protonation of the double bond to form an intermediate carbonium ion (Figure 20.18) or possibly attack by water or OH anion to produce a carbanion, followed by protonation.
20.11 ● Malate Dehydrogenase—Completing the Cycle
In the last step of the TCA cycle, L-malate is oxidized to oxaloacetate by malate dehydrogenase (Figure 20.19). This reaction is very endergonic, with a G° of 30 kJ/mol. Consequently, the concentration of oxaloacetate in the mitochondrial matrix is usually quite low (see the following example). The reaction, however, is pulled forward by the favorable citrate synthase reaction. Oxidation of malate is coupled to reduction of yet another molecule of NAD , the third one of the cycle. Counting the [FAD] reduced by succinate dehydrogenase, this makes the fourth coenzyme reduced through oxidation of a single acetate unit.
|
|
|
|
|
|
|
|
NADH |
|
|
|
|
|
|
|
|
|
|
|
|
|
NAD+ |
+ H+ |
|
|
|
|
|
|
|
|
OH |
|
|
|
|
O |
|
|
|
||||
|
|
|
|
|
COO– |
|
|
|
|
|
|
COO– |
||
H |
|
C |
|
|
Malate |
C |
|
|
||||||
|
|
|
|
|||||||||||
|
|
|
|
|
COO– |
|
dehydrogenase |
|
|
|
|
COO– |
||
H2C |
|
|
|
|
|
H2C |
|
|
||||||
|
|
NAD+ |
NADH |
|
|
|||||||||
|
L-Malate |
|
Oxaloacetate |
|||||||||||
|
|
|
+ H+ |
|||||||||||
FIGURE 20.19 ● The malate dehydrogenase reaction.


20.11 ● Malate Dehydrogenase—Completing the Cycle |
657 |
Steric Specificity for NAD of Various Pyridine Nucleotide-Linked Enzymes
|
|
|
Steric |
|
|||||
Dehydrogenase |
Source |
|
Specificity |
|
|||||
|
|
|
|
|
|
|
|
|
|
Alcohol (with ethanol) |
Yeast, Pseudomonas, liver, |
|
|
|
|
|
|
|
|
|
wheat germ |
|
|
|
|
|
|
|
|
Alcohol (with isopropyl alcohol) |
Yeast |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Acetaldehyde |
Liver |
|
|
|
|
|
|
|
|
L-Lactate |
Heart muscle, Lactobacillus |
|
HR |
|
|||||
|
|
|
|
|
|
|
|
||
L-Malate |
Pig heart, wheat germ |
|
|
|
|
|
|
|
|
D-Glycerate |
Spinach |
|
|
|
|
|
|
|
HR |
|
|
R |
|
N |
|||||
Dihydroorotate |
Zymobacterium oroticum |
|
|
|
|
|
|
|
HS |
-Glycerophosphate |
Muscle |
|
|
|
|
|
|
C |
O |
|
|
|
|
|
|||||
Glyceraldehyde-3-P |
Yeast, muscle |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|||
L-Glutamate |
Liver |
|
|
|
|
|
|
NH2 |
|
|
|
|
|
|
|
|
|||
D-Glucose |
Liver |
|
|
|
|
|
|
|
|
-Hydroxysteroid |
Pseudomonas |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
HS |
|
||||||
NADH cytochrome c reductase |
Rat liver mitochondria, |
|
|
||||||
|
pig heart |
|
|
|
|
|
|
|
|
NADPH transhydrogenase |
Pseudomonas |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
NADH diaphorase |
Pig heart |
|
|
|
|
|
|
|
|
L- -Hydroxybutyryl-CoA |
Heart muscle |
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
H |
|
|
|
|
|
O H |
|
|
CH3 |
C |
|
|
|
|
|
H |
+ |
CH3 C |
|||
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
C |
C |
H |
|
|
C |
C |
R N |
|
C |
|
|
R N |
C H |
C |
C |
|
H+ |
C |
C |
|
|
C |
|
|
C |
||
|
|
|
|
|
||
|
NH2 |
O |
|
|
NH2 |
O |
The stereospecificity of hydride transfer in dehydrogenases is a consequence of the asymmetric nature of the active site.
Adapted from Kaplan, N. O., 1960. In The Enzymes, vol. 3, p. 115, edited by Boyer, Lardy, and Myrbäck. New
York: Academic Press.

658 Chapter 20 ● The Tricarboxylic Acid Cycle
EXAMPLE
A typical intramitochondrial concentration of malate is 0.22 mM. If the [NAD ]/[NADH] ratio in mitochondria is 20 and if the malate dehydrogenase reaction is at equilibrium, calculate the intramitochondrial concentration of oxaloacetate at 25°C.
SOLUTION
For the malate dehydrogenase reaction,
Malate NAD 34 oxaloacetate NADH H with the value of G° being 30 kJ/mol. Then
G° RT ln K eq
[1]x(8.314 J mol.K) (298) ln [20][2.2 10 4]
30,000 J mol ln (x 4.4 10 3) 2478 J mol
12.1 ln (x 4.4 10 3)
x (5.6 10 6)(4.4 10 3) x [oxaloacetate] 0.024 M
Malate dehydrogenase is structurally and functionally similar to other dehydrogenases, notably lactate dehydrogenase (Figure 20.20). Both consist of alternating -sheet and -helical segments. Binding of NAD causes a conformational change in the 20-residue segment that connects the D and E strands of the -sheet. The change is triggered by an interaction between the adenosine phosphate moiety of NAD and an arginine residue in this loop region. Such a conformational change is consistent with an ordered single-displacement mechanism for NAD -dependent dehydrogenases (Chapter 14).
(b)
● (a) The structure of malate dehydrogenase. (b) The active site of malate dehydrogenase. Malate is shown in red; NAD is blue.

20.12 ● A Summary of the Cycle |
659 |
20.12 ● A Summary of the Cycle
The net reaction accomplished by the TCA cycle, as follows, shows two molecules of CO2, one ATP, and four reduced coenzymes produced per acetate group oxidized. The cycle is exergonic, with a net G° for one pass around the cycle of approximately 40 kJ/mol. Table 20.1 compares the G° values for the individual reactions with the overall G° for the net reaction.
Acetyl-CoA 3 NAD [FAD] ADP Pi 2 H2O 34
2 CO2 3 NADH 3 H [FADH2] ATP CoASHG° 40 kJ/mol
Glucose metabolized via glycolysis produces two molecules of pyruvate and thus two molecules of acetyl-CoA, which can enter the TCA cycle. Combining glycolysis and the TCA cycle gives the net reaction shown:
Glucose 2 H2O 10 NAD 2 [FAD] 4 ADP 4 Pi 34
6 CO2 10 NADH 10 H 2 [FADH2] 4 ATP
All six carbons of glucose are liberated as CO2, and a total of four molecules of ATP are formed thus far in substrate-level phosphorylations. The 12 reduced coenzymes produced up to this point can eventually produce a maximum of 34 molecules of ATP in the electron transport and oxidative phosphorylation pathways. A stoichiometric relationship for these subsequent processes is
NADH H 12 O2 3 ADP 3 Pi 34 NAD 3 ATP 4 H2O [FADH2] 12 O2 2 ADP 2 Pi 34 [FAD] 2 ATP 3 H2O
Thus, a total of 3 ATP per NADH and 2 ATP per FADH2 may be produced through the processes of electron transport and oxidative phosphorylation.
The Fate of the Carbon Atoms of Acetyl-CoA in the TCA Cycle
It is instructive to consider how the carbon atoms of a given acetate group are routed through several turns of the TCA cycle. As shown in Figure 20.21, neither of the carbon atoms of a labeled acetate unit is lost as CO2 in the first turn of the cycle. The CO2 evolved in any turn of the cycle derives from the carboxyl groups of the oxaloacetate acceptor (from the previous turn), not from incoming acetyl-CoA. On the other hand, succinate labeled on one end from the original labeled acetate forms two different labeled oxaloacetates. The carbonyl carbon of acetyl-CoA is evenly distributed between the two carboxyl carbons of oxaloacetate, and the labeled methyl carbon of incoming acetyl-CoA ends up evenly distributed between the methylene and carbonyl carbons of oxaloacetate.
When these labeled oxaloacetates enter a second turn of the cycle, both of the carboxyl carbons are lost as CO2, but the methylene and carbonyl carbons survive through the second turn. Thus, the methyl carbon of a labeled acetyl-CoA survives two full turns of the cycle. In the third turn of the cycle, one-half of the carbon from the original methyl group of acetyl-CoA has become one of the carboxyl carbons of oxaloacetate and is thus lost as CO2. In the fourth turn of the cycle, further “scrambling” results in loss of half of the remaining labeled carbon (one-fourth of the original methyl carbon label of acetyl-CoA), and so on.
It can be seen that the carbonyl and methyl carbons of labeled acetyl-CoA have very different fates in the TCA cycle. The carbonyl carbon survives the first turn intact but is completely lost in the second turn. The methyl carbon




 S CoA
S CoA







 Ketoglutarate
Ketoglutarate

 S CoA
S CoA



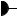


 Ketoglutarate
Ketoglutarate








 Ketoglutarate
Ketoglutarate