
- •Contents
- •Foreword
- •How to use this book
- •Advisory boards
- •Contributing writers
- •Contributing illustrators
- •What is an insect?
- •Evolution and systematics
- •Structure and function
- •Life history and reproduction
- •Ecology
- •Distribution and biogeography
- •Behavior
- •Social insects
- •Insects and humans
- •Conservation
- •Protura
- •Species accounts
- •Collembola
- •Species accounts
- •Diplura
- •Species accounts
- •Microcoryphia
- •Species accounts
- •Thysanura
- •Species accounts
- •Ephemeroptera
- •Species accounts
- •Odonata
- •Species accounts
- •Plecoptera
- •Species accounts
- •Blattodea
- •Species accounts
- •Isoptera
- •Species accounts
- •Mantodea
- •Species accounts
- •Grylloblattodea
- •Species accounts
- •Dermaptera
- •Species accounts
- •Orthoptera
- •Species accounts
- •Mantophasmatodea
- •Phasmida
- •Species accounts
- •Embioptera
- •Species accounts
- •Zoraptera
- •Species accounts
- •Psocoptera
- •Species accounts
- •Phthiraptera
- •Species accounts
- •Hemiptera
- •Species accounts
- •Thysanoptera
- •Species accounts
- •Megaloptera
- •Species accounts
- •Raphidioptera
- •Species accounts
- •Neuroptera
- •Species accounts
- •Coleoptera
- •Species accounts
- •Strepsiptera
- •Species accounts
- •Mecoptera
- •Species accounts
- •Siphonaptera
- •Species accounts
- •Diptera
- •Species accounts
- •Trichoptera
- •Species accounts
- •Lepidoptera
- •Species accounts
- •Hymenoptera
- •Species accounts
- •For further reading
- •Organizations
- •Contributors to the first edition
- •Glossary
- •Insects family list
- •A brief geologic history of animal life
- •Index
Order: Phthiraptera |
Vol. 3: Insects |
Species accounts
Elephant louse
Haematomyzus elephantis
FAMILY
Haematomyzidae
TAXONOMY
Haematomyzus elephantis Piaget, 1869. Type host: Loxodonta africana.
OTHER COMMON NAMES
None known.
PHYSICAL CHARACTERISTICS
Distinct triangular head with the pre-antennal region elongated into a long rostrum bearing a pair of outward-facing mandibles. Short, broad thorax without a sternal plate and long, slender legs with a single serrated claw.
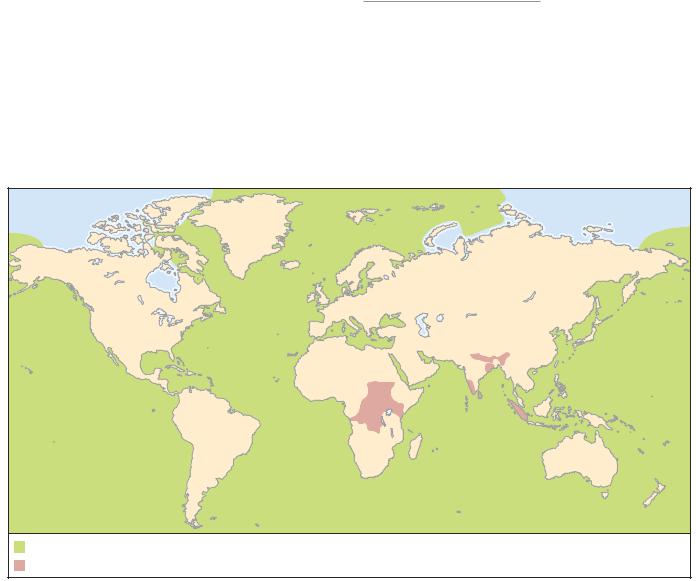
DISTRIBUTION
Restricted to the Indian and African elephant but not recorded throughout the host’s range and less common on African elephants.
HABITAT
Common on the more hairy regions, especially in folds of soft skin of juvenile elephants. They are most common on the ears, groin, or axilla (armpits) or at the base of the tail.
BEHAVIOR
Nothing is known.
FEEDING ECOLOGY AND DIET
Sharp, outward-facing mandibles rasp at the surface of the skin, causing blood to flow. The louse sucks the blood up through a median notch at the end of the rostrum.
REPRODUCTIVE BIOLOGY
Nothing is known.
CONSERVATION STATUS
Not listed by the IUCN but should be considered threatened in those areas where the host population is considered endangered.
SIGNIFICANCE TO HUMANS
None known.
Wandering seabird louse
Ancistrona vagelli
FAMILY
Menoponidae
TAXONOMY
Pediculus vagelli Fabricius, 1787. Type host: Fulmarus g. glacialis.
OTHER COMMON NAMES
None known.
Ancistrona vagelli
Haematomyzus elephantis
254 |
Grzimek’s Animal Life Encyclopedia |
Vol. 3: Insects
PHYSICAL CHARACTERISTICS
Distinguished by its large size (0.1–0.2 in, or 3–6 mm), large triangular or rectangular postnotum, characteristic gular processes, and the absence of setal brushes on the venter of the third femur and abdominal sternites.
DISTRIBUTION
Recorded from more than 45 species of seabird in the families Procellaridae and Hydrobatidae. These include species of fulmar, petrel, prion, and shearwater, with a combined distribution covering virtually every patch of seawater in the world.
HABITAT
Restricted to the host’s plumage, particularly regions surrounding the head and neck of the bird that are preened infrequently or are difficult to preen.
BEHAVIOR
Particularly vagile; hence its name. Capable of moving rapidly over the skin between feathers. This behavior may explain why this species has one of the widest host distributions of any louse in the suborder Amblycera.
FEEDING ECOLOGY AND DIET
Mainly soft feathers, particularly those close to the skin. As with most Amblycera, however, this probably is supplemented with traces of host blood, serum, and skin debris.
REPRODUCTIVE BIOLOGY
Nothing specific is known. As with most avian lice, the female is considerably larger than the male and always is found in greater numbers on the host. Eggs are cemented onto feather barbules and hatch within five to 10 days.
CONSERVATION STATUS
Not threatened. However, some populations of the species are restricted to rare or endangered hosts, such as the magenta pe-
Order: Phthiraptera
trel (Pterodroma magentae) and as such should be considered vulnerable.
SIGNIFICANCE TO HUMANS
None known.
Human head/body louse
Pediculus humanus
FAMILY
Pediculidae
TAXONOMY
Pediculus humanus Linnaeus, 1758. Type host: Homo sapiens.
OTHER COMMON NAMES
English: Cootie
PHYSICAL CHARACTERISTICS
Head with distinctive dark eyes. Abdomen elongate and lacking distinct tubercles. The head louse variant is typically 20% smaller than the body louse form.
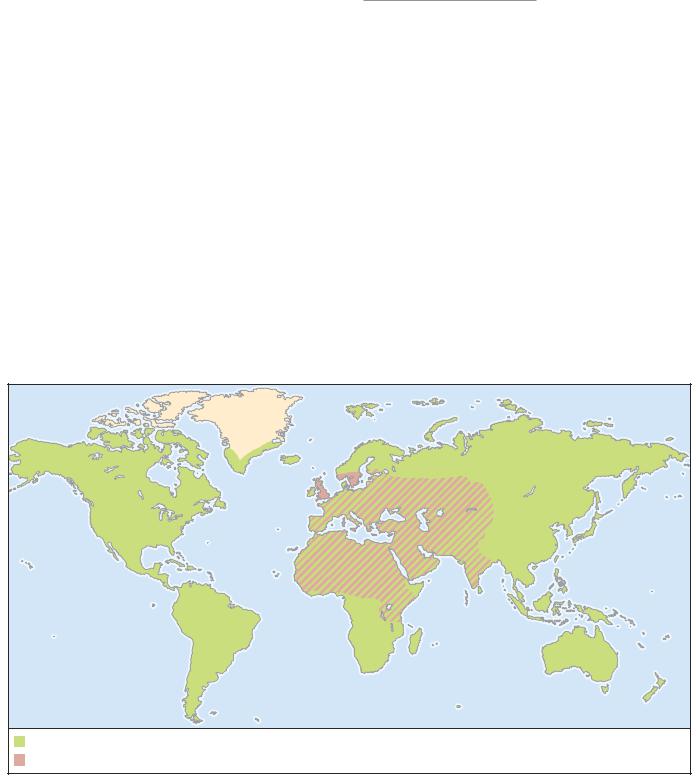
DISTRIBUTION
Worldwide. Lives as an ectoparasite on humans but also is recorded from gibbons and New World monkeys.
HABITAT
Two morphological variants exist that were once thought to be separate species or subspecies. The form commonly referred to as “head lice” is restricted to the human scalp, whereas the form referred to as “body lice” is restricted to clothing and the human torso.
Pediculus humanus
Columbicola columbae
Grzimek’s Animal Life Encyclopedia |
255 |

Order: Phthiraptera
BEHAVIOR
Human head and body lice are ecological variants of the same species, capable of adapting to the different ecological conditions on the human scalp, torso, or clothing. They are intermixed genetically, and their behavioral ecology has been studied extensively, as they are the vector for several important human diseases.
FEEDING ECOLOGY AND DIET
Both forms feed on blood, but their ecology differs between body morphs. Head lice feed at regular intervals every few hours, whereas body lice feed only once or twice per day during periods of host inactivity.
REPRODUCTIVE BIOLOGY
The head louse variant attaches eggs (nits) to the base of hair shafts, whereas body lice glue their eggs to projecting fibers of clothing on the host. Eggs typically hatch in five to seven days, and the nymphs reach maturity after another 10–12 days.
CONSERVATION STATUS
Not threatened. However, populations restricted to isolated human tribes and nonhuman primates should be considered vulnerable.
SIGNIFICANCE TO HUMANS
As the principal vector of epidemic typhus (Rickettsia prowazekii), the body louse variant was responsible for hundreds of millions of deaths up until the early 1900s. Since World War II, large outbreaks of typhus have occurred mainly in Africa, with reported cases coming predominantly from Burundi, Ethiopia, and Rwanda. The head louse variant is common in the Western World, with infection rates exceeding 20% reported from selected primary schools in Australia, the United Kingdom, and the United States. This form is not known to transmit louse-borne diseases in natural circumstances.
Slender pigeon louse
Columbicola columbae
FAMILY
Philopteridae
TAXONOMY
Pediculus columbae Linnaeus, 1758. Type host: Columba livia domestica.
Vol. 3: Insects
OTHER COMMON NAMES
None known.
PHYSICAL CHARACTERISTICS
A long, slender louse, readily distinguished from other ischnoceran genera by the presence of two bladelike dorsal setae on the anterior margin of the head.
DISTRIBUTION
This louse is restricted to four species of pigeon (Columba eversmanni, C. guinea, C. livia, and C. oenas), including the widely distributed common rock dove. The latter has been introduced throughout the world and has a cosmopolitan distribution, living commensally with humans in temperate and tropical areas. Louse distribution is assumed to mirror the distribution of the host. The distribution map accompanying this text shows only the host’s native distribution.
HABITAT
Restricted to the wing feathers of their hosts, either on the undersurface of the wing coverts or at the base of secondary feathers.
BEHAVIOR
Its slender shape allows the louse to live between the feather barbs. The edge of the barb is grasped with the mandibles and legs, protecting it from the preening activities of the host.
FEEDING ECOLOGY AND DIET
Feeds on the downy part of the feathers and may migrate from the wings to feed on the fluffy basal portions of body feathers.
REPRODUCTIVE BIOLOGY
Females deposit their eggs on the underside of the wing feathers, next to the pigeon’s body. Eggs are attached to a feather in the space between feather barbs and hatch between three and five days at 98.6°F (37°C).
CONSERVATION STATUS
Not threatened. However, populations present on Columba eversmanni (the pale-backed pigeon) should be considered vulnerable.
SIGNIFICANCE TO HUMANS
Used as a “model organism” by biologists to address questions on the evolution and ecology of host-parasite interactions.
Resources
Books
Hopkins, G. H. E., and T. Clay. A Checklist of the Genera and Species of Mallophaga. London: British Museum of Natural History, 1952.
Kim, K. C., H. D. Pratt, and C. J. Stojanovich. The Sucking Lice of North America. University Park: Pennsylvania State University Press, 1986.
Ledger, J. A. The Arthropod Parasites of Vertebrates in Africa South of the Sahara Vol. 4, Phthiraptera (Insecta). Johannesburg: South African Institute for Medical Research, 1980.
Palma, R. L., and S. C. Barker. “Phthiraptera.” In Psocoptera, Phthiraptera, Thysanoptera, edited by A. Wells. Zoological Catalogue of Australia, vol. 26. Melbourne, Australia:
CSIRO Publishing; 1996.
Price, Roger D., Ronald A. Hellenthal, Ricardo L. Palma, Kevin P. Johnson, and Dale H. Clayton. The Chewing Lice: World Checklist and Biological Overview. Illinois Natural History Survey Special Publication no. 24. Champaign-Urbana: Illinois Natural History Survey, 2003.
256 |
Grzimek’s Animal Life Encyclopedia |
Vol. 3: Insects
Resources
Periodicals
Clay, T. “Some Problems in the Evolution of a Group of Ectoparasites.” Evolution 3 (1949): 279–299.
— “The Amblycera (Phthiraptera: Insecta).” Bulletin of the British Museum (Natural History) Entomology 25 (1970): 73–98.
Durden, L. A, and G. G. Musser. “The Sucking Lice (Insecta, Anoplura) of the World: A Taxonomic Checklist with Records of Mammalian Hosts and Geographical Distributions.” Bulletin of the American Museum of Natural History 218 (1994): 1–90.
Price, M. A., and O. H. Graham. “Chewing and Sucking Lice as Parasites of Mammals and Birds.” USDA Agricultural Research Service Technical Bulletin 1849 (1997): 1–309.
Order: Phthiraptera
Smith, V. S. “Avian Louse Phylogeny (Phthiraptera: Ischnocera): A Cladistic Study Based Morphology.”
Zoological Journal of the Linnean Society 132 (2001): 81–144.
Other
“Tree of Life—Phthiraptera” [January 14, 2003].
<http://tolweb.org/tree/eukaryotes/animals/arthropoda/
hexapoda/phthiraptera/phthiraptera.html>.
“National Pediculosis Association” [January 14, 2003].
<http://www.headlice.org>.
“Phthiraptera Central.” November 8, 2002 [January 14, 2003].
<http://www.phthiraptera.org>.
“Phthiraptera Research” [January 14, 2003]. <http:// darwin.zoology.gla.ac.uk/~vsmith>.
Vincent S. Smith, PhD
Grzimek’s Animal Life Encyclopedia |
257 |
This page intentionally left blank
