
Multiple Bonds Between Metal Atoms / 06-X3M _ MX3 Compounds of Molybdenum and Tungsten
.pdf
X3MɓMX3 Compounds of Molybdenum and Tungsten 223
Chisholm and Hollandsworth
6.4
Fig. 6.8. Structure of Mo4(O)2(CH2SiMe3)8.
6.6M2X6L, M2X6L2 and Related Compounds
This class of compounds arises from the ability of the homoleptic X3MɓMX3 compounds to expand their coordination sphere either by direct association with a Lewis base or by virtue of the fact that one X ligand can be replaced by a uninegative bidentate group. A notable feature of this class of compounds is the drive to form symmetrically-substituted compounds. Each metal atom tends to be surrounded by an identical set of ligands and when this does not occur, each metal atom at least enjoys the same coordination number. Because of this observation, particular attention has been given to the following exceptions.
6.6.1 Mo2(CH2Ph)2(OPri)4(PMe3) and [Mo2(OR)7]-
The addition of PMe3 to 1,2-Mo2(CH2Ph)2(OPri)4 in hydrocarbon solvents was studied in great detail by 31P and 1H VT NMR spectroscopy and revealed the facile nature of benzyl for alkoxide exchange between metal centers.146 The symmetrically substituted compound Mo2(CH2Ph)2(OPri)4(PMe3)2 is formed in this reaction and is thermodynamically favored like its structurally characterized analog 1,2-Mo2(CH2Ph)2(OPri)4(dmpm). However, the unsymmetrically substituted compound (PMe3)(PhCH2)2(PriO)MoɓMo(OPri)3 is present at room temperature and was structurally characterized, and it is shown in Fig. 6.9. In solution, this compound is labile to PMe3 dissociation to reform the symmetrically substituted ethane-like compound 1,2-Mo2(CH2Ph)2(OPri)4. Given the kinetic persistence of 1,1- and 1,2-M2X2Y4 isomers, the significance of this Lewis base facilitated migration of groups between the metal centers becomes apparent.

224Multiple Bonds Between Metal Atoms Chapter 6
Fig. 6.9. Structure of (PMe3)(PhCH2)2(PriO)MoɓMo(OPri)3.
The addition of KOR to M2(OR)6 compounds was studied for M = Mo and W and R = But, Pri and CH2But.147 In the presence of 18-crown-6, the K+ (crown) salts of the anions M2(OR)7- and M2(OR)82- were isolated. Most significantly, the M2(OR)7- anions contained a single bridging alkoxide group for M = Mo and R = Pri. The Mo>Mo distance of 2.22 Å was only slightly longer than that found in Mo2(OPri)6. In solution, the anionic alkoxide is fluxional on the NMR time scale. Even at -80 ºC only one set of alkoxide resonances is visible. Again this attests to the facility of ligand transfer between the metal atoms in a M2X6L type of complex.
6.6.2 M2(OR)6L2 compounds and their congeners
In the reactions between M2(NMe2)6 compounds and alcohols, the dimethylamine that is liberated can coordinate to the dinuclear center to give dimethylamine adducts of the type M2(OR)6(HNMe2)2.58,148 The HNMe2 ligands are kinetically labile and may be readily replaced by other Lewis bases. Thus, in the reaction between W2(NMe2)6 and isopropanol in the presence of pyridine, the black crystalline compound W2(OPri)6(py)2 is formed149 whereas in a related reaction involving ethanol in the presence of en'' ligands, W2(OEt)6(Me(H)NCH2CH2N(H)Me) is formed.58 Even sterically bulky alkoxide ligands such as those present in W2(OBut)6 and W2(OCMe2CF3)6 will undergo reversible Lewis base association reactions150 in solution with pyridine, 4-methylpyridine and isocyanides:
M2(OR)6 +2L M2(OR)6L2
The geometries of these M2(OR)6L2 complexes are largely determined by steric factors. The two square planar M(OR)3L units are united by a M>M bond that is typically only 0.05 Å longer than in the unligated complex. Staggered geometries about the M>M bond are common but amine to alkoxide hydrogen bonding can favor an eclipsed geometry. Structural data for such compounds are given in Table 6.5.
Table 6.5. Compounds of the form X4MɓMX4 containing intramolecular hydrogen-bonding
Compound |
Donor |
Acceptor |
M–Ma |
ref. |
cis,cis-Mo2(OC6F5)4(NMe2)2(HNMe2)2 |
NHR2 |
OR |
2.22 |
151 |
Mo2[OCH(CF3)2]5(NMe2)(HNMe2)2 |
NHR2 |
OR |
2.24 |
152 |
Mo2(OBut)4(NHPh)2(NH2Ph)2 |
NHR/ NHR2 |
OR |
2.25 |
153 |
trans-W2Cl4(NHCy)2(NH2Cy)2 |
NHR |
Cl |
2.29 |
154 |
trans-W2Cl4(NHBut)2(NH2But)2 |
NHR |
Cl |
2.29 |
50,155 |
W2Cl3(NHBut)2(NH2But)(PPh2NPOPh2) |
NHR |
Cl |
2.30 |
154 |
trans-W2Cl4(NHBut)2(PMe3)2 |
NHR |
Cl |
2.31 |
156 |
trans-W2Cl4(NHBut)2(PMe3)2 |
NHR |
Cl |
2.31 |
156 |
trans-W2Cl4(NHBut)2(PMe2Ph)2 |
NHR |
Cl |
2.31 |
156 |

X3MɓMX3 Compounds of Molybdenum and Tungsten 225
|
|
Chisholm and Hollandsworth |
||
trans-W2Cl4(NHEt)2(NH2Et)2 |
NHR |
Cl |
2.31 |
155,157 |
trans-W2Cl4(NHBut)2(PPr3)2 |
NHR |
Cl |
2.32 |
156 |
cis,cis-W2Cl4(NHBut)2(dmpm) |
NHR |
Cl |
2.32 |
158 |
cis-W2Cl4(NHCy)2(PMe3)2 |
NHR |
Cl |
2.32 |
154 |
cis-W2Cl4(NHBut)2(PMe3)2 |
NHR |
Cl |
2.32 |
154 |
1,1,2-W2Cl3(OBut)3(NHMe2)2 |
NHR |
OR |
2.32 |
159 |
W2(OBut)4(NHPh)2(NH2Ph)2 |
NHR/ NHR2 |
OR |
2.32 |
153 |
cis-W2Cl4(NHBut)2(PMe3)2 |
NHR |
Cl |
2.33 |
156 |
cis,cis-W2Cl4(NHBut)2(dmpe) |
NHR |
Cl |
2.33 |
158 |
cis,cis-W2Cl4(NHBut)2(dppm) |
NHR |
Cl |
2.33 |
158 |
cis,cis-W2(OC6F5)4(NMe2)2(HNMe2)2 |
NHR2 |
OR |
2.34 |
b |
W2(OPri)6(HNMe2)2 |
NHR2 |
OR |
2.34 |
58 |
cis,cis-W2Cl4(NHBut)2(dppe) |
NHR |
Cl |
2.35 |
158 |
a Å, ± 0.01 Å
bM. H. Chisholm, J. C. Gallucci, C. B. Hollandsworth, unpublished crystal structure of W2(OC6F5)4(NMe2)2- (HNMe2)2.
The dynamic equilibrium is slow enough to be monitored by variable temperature NMR studies which reveal the cooperative nature of the binding and releasing of the ligands, L. At higher temperatures, entropy favors the unligated M2(OR)6 compounds whereas, at low temperatures, the enthalpy of ligation dominates. The position of equilibrium is very sensitive to steric factors associated with the alkoxide and the Lewis base. Also the relative elec- tron-donating properties of the alkoxide play a significant role following the donicity order Me3CO > Me2CF3CO > Me(CF3)2CO. Ease of adduct formation follows the inverse order of alkoxide donicity. The tertiary phosphine adducts W2(OCH2CMe3)6(PMe3)2 and W2(OCH2CMe3)6- (Me2PCH2CH2PMe2) show interesting 31P NMR spectra as a result of the dynamic equilbria just described and also because of the presence of 183W, I = ½, c. 14% natural abundance, which gives rise to a satellite spectrum reflecting the magnetically different 31P nuclei in the mixed 183W>W isotopomer. Addition of alkoxide anions to M2(OR)6 compounds has also been noted to give M2(OR)82- anions supported by lithium, potassium, or H2NMe2+ cations.147,160 For the latter, the presence of excess, acidic alcohol, causes formation of (H2NMe2)(OR) from the reaction of liberated HNMe2 and ROH. The salt can then add to W2(OR)6 to give (H2NMe2)2[W2(OR)8] species in which the H2NMe2+ cations form strong hydrogen bonding interactions to the alkoxide ligands.
A pair of four-coordinate, triply-bonded dimetal centers also arises when two alkoxide ligands are replaced by a bidentate chelating ligand such as a carboxylate or acac ligand. In the case of M2(OR)4(acac)2 compounds, the acac acts as chelating group to each metal center resulting in unbridged four-coordinated metal atoms.161 A similar unbridged MM bond was seen in the W2R2(OPri)2(But-acac)2 compounds, where R = Et, Ph, CH2Ph or Bu, and But-acac = 2,5-di-tert-butylpentanedienylate.162
Insertion of CO2 into the M-OR bond occurs reversibly for M2(OR)6 compounds to give the alkylcarbonate M2(OR)4(O2COR)2 compounds163 which like their carboxylate analogs164 have four coordinate metal centers and two mutually cis, bridging carboxylates.
A related double insertion also occurs in the reactions of M2(OR)6 and organic isocyanates and the compounds Mo2(OPri)4(N(Ph)C(O)OPri)2 and W2(OBut)4(N(Ph)C(O)OBut)2 were structurally characterized.165 The structure of the tungsten compound is shown in Fig. 6.10. Here there is a trans O–W–N arrangement such that each metal is in an equivalent environment. In the case of the Mo2-containing compound, the bridging groups are cis but symmetrically disposed so that each metal center forms three M–O bonds and one M–N bond.165 In a subsequent

226Multiple Bonds Between Metal Atoms Chapter 6
study,166 it was found that an initial adduct was formed in which the phenylisocyanate molecule bridged to two metal centers and the product resulting from subsequent insertion into a metalalkoxide bond was isolated as the PMe3 adduct. These structures are depicted in 6.5 and 6.6.
Fig. 6.10. Structure of W2(OBut)4(N(Ph)C(O)OBut)2.
6.5 |
6.6 |
6.6.3 Amido-containing compounds
The homoleptic M2(NMe2)6 compounds do not form Lewis base adducts, although once replacement of the NMe2 groups occurs by less electron donating groups, Lewis base adduct formation is common. For example, M2Cl2(NMe2)4 compounds react with tertiary phosphine ligands and a variety of mixed chloroamido phosphine complexes have been characterized including W2Cl2(NMe2)4(dmpm), W2Cl3(NMe2)3(PMe2Ph)2, and W2Cl4(NMe2)2(PMe2Ph)2 by single crystal X-ray diffraction studies.167 Notable within this series are the W–N bond distances which decrease as more Cl groups are introduced to the tungsten center. Also the rotational barrier about the W–N bonds reflects these changes and increases with increasing NMe2 p/ to Wd/ donation. Similarly in the reactions of fluoroalkoxides, mixed amido/ alkoxide/amine complexes have often been isolated and a particularly interesting series of compounds was isolated in a study of the reactions between Mo2(NMe2)6 and pentafluorophenol, C6F5OH.151 These include Mo2(OC6F5)4(NMe2)2(HNMe2)2, Mo2(OC6F5)5(NMe2)(HNMe2)2, and the MoMo quadruply bonded complex Mo2(OC6F5)4(HNMe2)4 as well as (Me2NH2)[Mo2(OC6F5)6(HNMe2)2], which has a formal bond order of 3.5. Aside from the occurrence of redox reaction products in what is seemingly a simple alcoholysis reaction, the product Mo2(OC6F5)5(NMe2)(HNMe2)2 warrants special note. The structure of this compound is shown in Fig. 6.11.
The two four-coordinate metal centers are staggered but, surprisingly, four phenolate groups are bonded to one Mo atom while the other has one phenolate and one NMe2 group, along with two dimethylamines. The (Mo>Mo)6+ center is thus polar having one Mo4+ and one Mo2+ center. The Mo>Mo distance of 2.214(1) Å is unexceptional and consistent with a MoMo triple bond. It can be thought of as having one dative component from the Mo2+ center to the Mo4+ akin to that in carbon monoxide where oxygen provides four of the six electrons employed to form the triple bond.

X3MɓMX3 Compounds of Molybdenum and Tungsten 227
Chisholm and Hollandsworth
Fig. 6.11. Structure of Mo2(OC6F5)5(NMe2)(HNMe2)2.
Reactions with reduced tungsten halides (as in THF solutions of NaW2Cl7),6 with bulky primary amines have also been shown to give mixed chloroamido amine complexes such as W2Cl4(N(H)But)2(H2NBut)2.50 Also in a rare example of the replacement of an alkoxide by an amide, it was found that M2(OBut)6 compounds react with aniline to form the mixed amide/ alkoxide/amine complexes M2(OBut)4(N(H)Ph)2(N(H)2Ph)2 with the liberation of ButOH.48 In these compounds, significant N–H Cl or N–H O hydrogen bonds exist across the M>M bond and thus favor the eclipsed geometry, as noted in Table 6.5.
Reactions involving 1,2-M2Cl2(NMe2)4 wherein the chloride ligands are replaced by bidentate uninegative ligands gave compounds containing 2-oxy-6-methylpyridine,168 1,3- di-p-tolyltriazenido,169 and 6-methyl-2-pyridylmethyl.170 Ditolyltriazene reacts with both Mo2(NMe2)6169 and 1,2-Mo2Me2(NMe2)4 to replace one NMe2 group from each Mo atom but the structures of the products are quite different. In Mo2(NMe2)4(ArNNNAr)2, the triazenido ligands are chelating whereas in Mo2Me2(NMe2)2(ArNNNAr)2, they bridge the (Mo>Mo)6+ bond. These two structures are shown in Fig. 6.12.
Fig. 6.12. Structures of Mo2(NMe2)4(δ2-ArNNNAr)2 and Mo2Me2(NMe2)2(µ-ArNNNAr)2.
W2(NMe2)6 and 1,3-diphenyltriazene react similarly to give W2(NMe2)4(PhNNNPh)2 which is structurally similar to its molybdenum analog. However, Mo2R2(NMe2)4 and W2R2(NMe2)4 compounds where R = ethyl and benzyl react quite differently with 1,3-diaryl triazenes.77, 118 The molybdenum compounds undergo reductive elimination to yield the MoMo quadruply bonded compound Mo2(ArNNNAr)4, whereas the tungsten compounds give W2R2(NMe2)2(ArNNNAr)2, which are analogs of Mo2Me2(NMe2)2(ArNNNAr)2 shown in Fig. 6.12. Similar reactions occur with M2R2(NMe2)4 compounds and carbon dioxide. The molybdenum compounds are more susceptible to reductive elimination via alkyl group disproportionation as noted earlier. The benzyl complexes undergo reductive elimination by a radical process.76

228Multiple Bonds Between Metal Atoms Chapter 6
6.6.4 Mo2Br2(CHSiMe3)2(PMe3)4
The compounds M2(CH2R)6 where R = CMe3, SiMe3 and Ph do not react with Lewis bases but the compound 1,2-Mo2Br2(CH2SiMe3)4 does. The addition of PMe3 induces alkane elimination via _-hydrogen activation and leads to an unusual dinuclear bis-alkylidene complex, Mo2Br2(CHSiMe3)2(PMe3)4.171,172 In the solid-state, this complex possesses C2 symmetry. The PMe3 ligands are mutually trans and the central Mo2Br2C2P4 skeleton is eclipsed as depicted in 6.7. The HCSi planes are aligned with the (Mo>Mo)6+ axis such that the carbene-Mo /-bond does not compete with the MoMo /-bond. The (Mo>Mo)6+ distance 2.276(1) Å is well within the normal range for Mo>Mo bonds.
6.7
6.6.5 Calix[4]arene complexes
Reactions between p-tert-butylcalix[4]arenes and M2(NMe2)6 or M2(OR)6 compounds leads to the formation of products where each metal is bonded to four oxygen atoms. Interestingly, in these reactions kinetic products of substitution were shown to have the calix[4]arene spanning the Mo>Mo bond. However, these compounds isomerized to the thermodynamic products upon heating in the presence of donor ligands such as pyridine as shown in Scheme 6.3 where ʇ represents Ν2NMe2+ hydrogen-bonded to the respective calixarene.173, 174
Scheme 6.3. Some reactions of M2(calixarene)2 compounds.
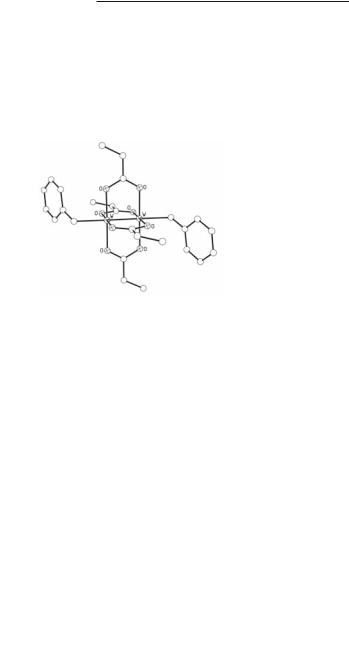
X3MɓMX3 Compounds of Molybdenum and Tungsten 229
Chisholm and Hollandsworth
6.7Triple Bonds Uniting Fiveand Six-Coordinate Metal Atoms
This is a small but interesting group of compounds. As noted earlier, there are compounds of the type W2R2(O2CMe3)4 where R = CH3, CH2Ph and CH2CMe3117,119-120 and Mo2(CH2CMe3)2(O2CMe)4122 that have very short MM distances comparable to MM quadruple bond distances. They have the ubiquitous paddlewheel geometry with additional axial alkyl ligation as seen for W2(CH2Ph)2(O2CEt)4 in Fig. 6.13.
Fig. 6.13. Structure of W2(CH2Ph)2(O2CEt)4.
A similar geometry is seen in the compounds M2(hpp)4Cl2 which are formed either by oxidation of the highly reducing M2(hpp)4 complexes or from the melt reaction involving M2Cl2(NMe2)4 and > 4 equivalents of Hhpp.175,176 These are noteworthy for having abnormally long M–Cl axial bonds of 3 Å. The former compounds, M2R2(O2CR')4, share a valence MO configuration MM /4β2 and the latter MM /4μ2, where the HOMO is MM μ- bonding and M–Cl μ* in character. This contrasts with the structure seen for W2Me2(O2CNEt2)4 where each metal atom forms five bonds in a pentagonal plane.108 Here the MM bonding configuration is μ2/4. However, subtle factors can induce a transformation from one structure to another as seen in the replacement of two acetate ligands by dithiocarbamate ligands in the compound W2(CH2CMe3)2(O2CMe)2(S2CNEt2)2.121 The latter has a WW bond of configuration μ2/4. An analysis of the frontier molecular orbitals indicated there should be no significant electronic barrier to the interconversion of these two structures, 6.8 and 6.9, and furthermore, that the nitrogen lone pairs in R2NCO21- and R2NCS21- ligands had a destabilizing influence on the /4β2 triple bond as in 6.8 thus favoring the μ2/4 configuration seen in 6.9.121
6.8 |
6.9 |
Finally, for metal atoms forming six bonds to ligands as in W2(O2CBut)628 and W2(O2CNMe2)6,108 there is only one possibility, namely that five bonds are formed in the xy plane and one additional bond along the (W>W)6+ axis. In these compounds, the EAN rule is satisfied and the triple bond is of configuration μ2/4. NMR spectroscopy indicates that these molecules are fluxional on the NMR time scale. To these structural motifs we can add the more common geometries for dinuclear metal complexes, namely edge-shared and face-shared

230Multiple Bonds Between Metal Atoms Chapter 6
bioctahedral which too can exist in equilibrium.177 This is further testimony to the remarkable coordination modes available to the M26+ unit.178
6.8Redox Reactions at the M26+ Unit
In 1979, Chisholm speculated about redox reactions of (M>M)6+ complexes and anticipated that these compounds should enter into redox reactions wherein the M>M bond order was systematically changed. Moreover, it was suggested that the dinuclear center could act as a template for catalytic reactions.179 As has been noted already, 1,2-dialkyl and -diaryl compounds were found to enter into reductive elimination reactions leading to the formation of MM quadruple bonds. The first examples of the oxidative conversion of a MM triple bond to a double or single bond are shown180 in the following reactions:
Mo2(OPri)6 + PriOOPri Α Mo2(OPri)8
Mo2(OPri)6 + 2X2 Α Mo2(OPri)6X4, where X = Cl, Br and I
Subsequently, it was shown that the octaalkoxide anions [M2(OR)8]2- could be cleanly converted to M2(OR)8 compounds:
K2M2(OR)8 + PPh3Br2 Α M2(OR)8 + 2KBr + PPh3
The latter reaction afforded access to W2(OCH2But)8, with a formal W=W double bond, which has now been shown to have an extensive organometallic chemistry.181-184
Oxidative addition reactions invariably led to bridge formation and the structure of the Mo2(OPri)847 and Mo2(OPri)6X4 compounds180 are shown schematically in 6.10 and 6.11. The related W2(OCH2But)8 compound is polymeric and is believed to have an extended chain structure of face sharing (W=W)8+ units linked by alkoxide bridges.
6.10 |
6.11 |
In an equatorial-axial bridged bipyramid, the M=M bond of about 2.5 Å can be formulated as having a μ- and a β- component but lacking a / component. In a pair of d1-d1 edge-sharing octahedra, the M–M single bond of length c. 2.7 Å is of μ2 origin, being formed from one of the t2g-t2g interactions.
Alcohols have also been found to oxidatively add to W2(OR)6 compounds to give hydridobridged structures such as that seen in [W2(µ-H)OPri)7]2.55,60 In the solid state, this compound contains a chain of four tungsten atoms, and the WW distances alternate between short, long, and short (2.45, 3.30 and 2.45 Å, respectively). This is consistent with the view that two confacial (W=W)8+ units are linked together by a pair of alkoxide bridges. It is intriguing that this molecule is fluxional on the NMR time-scale giving rise to only one type of alkoxide signal even at -80 ºC. The hydride signal appears downfield at about 20 ppm and is flanked by satellites due to coupling to two equivalent 183W nuclei. The tetranuclear structure of [W2(H)(OPri)7]2 is readily broken by the addition of Lewis bases or NaOR in diglyme. Consequently, Na[W2(H)(OPri)8] has been structurally characterized as the diglyme adduct.185 Although the reaction pathway leading to the oxidative addition of alcohol was a matter of considerable discussion, it was eventually argued that it is a base promoted addition.185

X3MɓMX3 Compounds of Molybdenum and Tungsten 231
Chisholm and Hollandsworth
_-Diketones R'C(O)C(O)R' were also found to react with W2(OR)6 compounds to give W–W singly bonded complexes W2(OR)6(OC(R')C(R')O)2 with W–W distances of c. 2.75 Å when R = But or Pri and R' = Me, Ph and p-tolyl.186 The _-diketone ligands are essentially reduced to diolates and chelate to the metal center.
In a similar manner Mo2(OR)6 compounds (R = Pri and CH2But) and W2(OPri)6(py)2 were found to react with 9,10-phenanthrenequinone and tetrachloro-1,2-benzoquinone to give (M–M)10+ units.95 Also, it was found that 1,4-diisopropyl-1,4-diazobutadiene adds to give the (M=M)8+ complex Mo2(OPri)6(PriNCHCHNPri)2 along with Mo2(OPri)5(PriNCHCHNPri)2.187 Mo2(OR)6 compounds and arylazides react to give imido compounds such as [Mo(OBut)2(NAr)(µ-NAr)]2 with complete loss of the MM bond and loss of one alkoxide ligand per metal atom.188 Diaryldiazoalkanes, Ph2CN2 react with M2(OR)6 to give Mo2(OPri)6(N2CPh2)2(py) and W2(OBut)6(N2CPh2)2. In each compound, the diazoalkane is reduced to a hydrazone ligand, N2CPh22-. In the tungsten compound, there is a fused trigonal bipyramidal geometry with a pair of bridging N2CPh2 ligands and a WW bond length of 2.67 Å. However, in the Mo structure, there are terminal N–N=CAr2 nitrene type ligands and
three bridging alkoxides spanning a MoMo bond of distance 2.66 Å.
The compounds M2(OR)6 undergo facile reactions with dry O2 and for M = Mo, the reaction is complex and dependent on the nature of R. For tungsten, the only observed product is W2O3(OBut)6 of unknown structure.189 For Mo2(OBut)6, the product was a thermally sensitive, yellow, volatile liquid MoO2(OBut)2.190 For Mo2(OR)6 compounds where R = Pri and CH2But, a more complex reaction sequence was observed and a variety of products were isolated from careful studies of O2 uptake.191 These included MoO2(OR)2(bpy), MoO(OR)4, Mo3(O)(OR)10, Mo4O4(OR)4(py)4 and Mo6O10(OR)12. The green, oxocapped triangular Mo4+-containing clusters, Mo3(µ3-O)(µ3-OR)(µ2-OR)3(OR)6, where R = Pri or CH2But, were shown192 to be formed by the following reaction:
M2(OR)6 + MO(OR)4 Α M3(O)(OR)10
This reaction proved quite general for both Mo and W when R = Pri and CH2But and the analogous, mixed metal MoW2 and Mo2W containing clusters were also prepared in this way.193,194 In a subsequent study, an imido capped triangular cluster W3(µ3-NH)(OPri)10 was isolated and was almost certainly formed in a manner similar to that shown above.
Reactions involving P4 and W2(OR)6 compounds yielded products derived from cleavage of the W>W bond: W(δ3-P3)(OCH2But)3(HNMe2)195 and W3(µ3-P)(OCH2But)9196 along with evidence of the phosphide (ButO)3W>P. This evidence was provided by a unique trapping experiment employing Cr(CO)5(THF).197
The compounds Mo2(OR)6 react with nitric oxide to give products where the M>M bond is cleaved and the compounds [Mo(OPri)3NO]2198 and W(OBut)3(NO)(py)199 were structurally characterized and shown to have the molecular forms depicted in 6.12 and 6.13 below.
6.12 |
6.13 |

232Multiple Bonds Between Metal Atoms Chapter 6
In both nitrosyl-adduct structures, the metal adopts a trigonal bipyramidal coordination environment with the linear nitrosyl ligand in an axial site. The M–N bond is depicted as a triple bond to emphasize that in this reaction with NO, the M>M bond is formally replaced by two M>N bonds. The compounds show extremely low values of ι(NO) as a result of extensive metal d/ to NO/* back-bonding with ι(NO) = 1640 cm-1 (M = Mo) and 1555 cm-1 (M = W). Moreover, the M–N distances, c. 1.74 Å are comparable to those seen in the compounds (ButO)3W>N200 and (ButO)3Mo>N.200,201
In contrast to these reactions that give nitrosyl derivatives, the compound W2(OSiMe2But)6 reacts with NO to produce oxotungsten compounds: WO(SiMe2But)4 and WO2(OSiMe2But)2.202 The same products are formed in reactions involving N2O. Evidently, in the reactions involving NO and W2(OSiMe2But)6, N–N bond formation occurs leading to O atom transfer and N2 liberation.
There are also various reactions that lead to complete loss of the MM bond as a result of redox reactions. For example, W2(OBut)6 reacts with nitrosobenzene and nitrobenzene to give the oxoimido tungsten complex (ButO)2(PhN)W(µ-O)(µ-OBut)2W(OBut)2(NPh).203 With bpy, M2(OR)6 compounds give products of redox disproportionation. The Mo(OPri)2(bpy)2 compound was shown to be an interesting molecule containing the d2-cis-MoO2N4 core. With an excess of aryl or t-butylisocyanide ligands or carbon monoxide, M(CNR)6 or M(CO)6 compounds are formed along with M6+ metal alkoxides.204 This provides a very efficient preparation of labeled M(CO)6 compounds in reactions employing 13CO or C18O. The latter are only sparingly soluble in alkane solvents and so are readily separated from the other more soluble transition metal alkoxide products.
6.9Organometallic Chemistry of M2(OR)6 and Related Compounds
Many of the reactions described in this section can be viewed as a redox reactions between /-acceptor, reducible organic molecules and the electron donating (M>M)6+ center. The presence of alkoxides or related /-donor ligands is ideal as the donor and steric properties can be modified in subtle ways. The flexible M–O–C angle allows for /-buffering and the ability of the alkoxide ligands to go between terminal and bridging positions make the (M>M)6+ center a remarkably responsive template for substrate binding and activation.205
6.9.1 Carbonyl adducts and their products
Carbon monoxide adds reversibly to Mo2(OBut)6 to form a 1:1 adduct206 while tungsten forms W2(OBut)6(CO) as a more kinetically persistent adduct.207 Both compounds adopt a common structure having a carbonyl ligand bridging two metal atoms that are in a square pyramidal environment with the M–C bond in the axial position shown in 6.14. The most notable feature of these monocarbonyls is the low value of ι(CO): 1575 cm-1 (M = W) and 1625 cm-1 (M = Mo). Hence in 6.14, the C–O and MM bonds are shown as double bonds [M=M = 2.50(1) Å (for M = Mo), 2.53(1) Å (for M = W), and C=O = 1.25 Å], and these compounds can be viewed as inorganic analogs of cyclopropenone.208,209
6.14
