
Multiple Bonds Between Metal Atoms / 11-Iron, Cobalt and Iridium Compounds
.pdf
11
Iron, Cobalt and
Iridium Compounds
Carlos A. Murillo,
Texas A&M University
11.1 General Remarks
There is a rich and extensive chemistry of the group 8 element ruthenium (see Chapter 9) which has a large number of Ru2n+ paddlewheel compounds, n = 4, 5, and 6. For the heaviest of the elements in this group (Os) there is also a substantial number of compounds having Os–Os bond orders of 2, 2.5 and 3 (see Chapter 10). However, no parallel in the chemistry of iron has been found yet.
Likewise, the extensive chemistry that has been discovered for metal–metal bonded Rh24+ and Rh25+ species that contain L4MML4 and L5MML5 structures based upon planar ML4 or square pyramidal ML5 geometries has led to the expectation that related, isoelectronic compounds of the group 9 elements Co and Ir should exist. However, the number of d7–d7 compounds of these elements is very limited. In this chapter we will focus our attention primarily on dimetal complexes for which each metal unit possesses a square planar configuration and the two square planes (with or without additional axial ligands) are parallel to each other and analogous metal–metal bonded compounds with two parallel triangular planes.
11.2 Di-iron Compounds
Compounds with Fe–Fe bonds without /-donor ligands, such as carbonyl, are scarce. To date, there is one family of paddlewheel compounds in which the presence of Fe–Fe bonds is unmistakable. The first such compound was initially prepared in low yield by reacting the diphenylformamidine-containing FeII compound FeCl2(HDPhF)2 and butyllithium which produces an unusual trigonal paddlewheel (also referred as a trigonal lantern) complex Fe2(DPhF)3 having an Fe23+ core and a very short Fe–Fe bond distance of 2.2318(8) Å.1 This distance is 0.25 Å shorter than that of 2.48 Å found in metallic iron. The reduction of the iron atom presumably proceeds through the attachment of a butyl group to the coordinatively unsaturated metal center followed by `-elimination. The hydride ion which remains could react with a coordinated HDPhF molecule to produce the corresponding bridging anion. An improved synthesis has been devised by adding the hydride reducing agent, NaEt3BH, before the deprotonating agent methyllithium.2 The net reaction is:
2FeCl2(HDPhF)2 + NaEt3BH + 4LiMe Α
Fe2(DPhF)3 + LiDPhF + 3LiCl + NaCl + ½H2 + BEt3 + 4CH4
447
448Multiple Bonds Between Metal Atoms Chapter 11
In this manner the analogous benzamidinate compound Fe2(DPhBz)3 has been made. This has an even shorter Fe–Fe bond of 2.198(2) Å. Indeed this is the shortest Fe–Fe distance in any iron containing compound. These compounds are the first paddlewheel complexes having an M23+ core in which each metal atom has a formal oxidation number of +1.5.
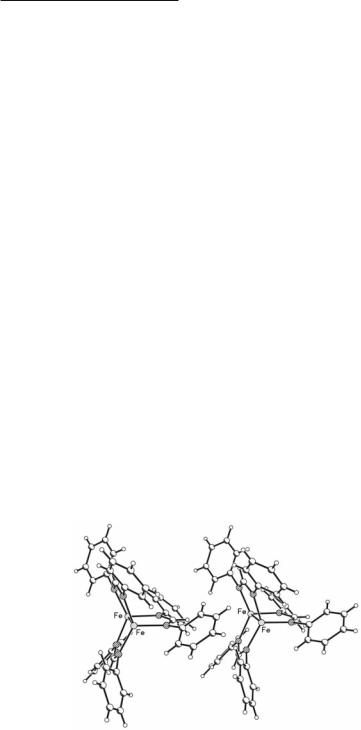
The core of these compounds, which also has Co analogs (see Section 11.3.2), is represented in 11.1. The molecular structures of the two compounds show that there are three amidinate bridges spanning the Fe23+ unit. In Fe2(DPhF)3, the formamidinato groups are not evenly distributed around the iron–iron line segment. One of the ring–ring dihedral angles, _, is opened (132.6º) while the other two are compressed (116.2 and 111.2º) relative to the ideal 120º. Thus the core of this molecule can be described as having C2v symmetry. For the benzamidinate complex Fe2(DPhBz)3 there is no distortion and the core has virtual D3h symmmetry. The reason for the distortion in Fe2(DPhF)3 has been attributed to the packing of the molecules in the crystal. This is shown in Fig. 11.1. The molecules are aligned along a crystallographic two-fold axis in such a way that two of the hydrogen atoms of two phenyl rings of adjacent molecules point toward the faces of phenyl rings of adjacent molecules, leading to a restrained packing arrangement that is accommodated by the open intramolecular dihedral angle. Theoretical calculations are consistent with this explanation as they indicate that the total energy of the ground state for the model compound Fe2(HNCHNH)3 does not change significantly as a function of the dihedral angle.3 A very detailed study of electron density maps ruled out the existence of any other species, such as a hydride ion, contributing to the distortion.
11.1
Fig. 11.1. Packing of Fe2(DPhF)3 molecules along the b axis which coincides with a crystallographic two-fold axis. In a given molecule, two DPhF ligands, related to each other by the two-fold axis, are pushed apart by Van der Waals contacts breaking the ideal D3h symmetry.
Iron, Cobalt and Iridium Compounds 449
Murillo
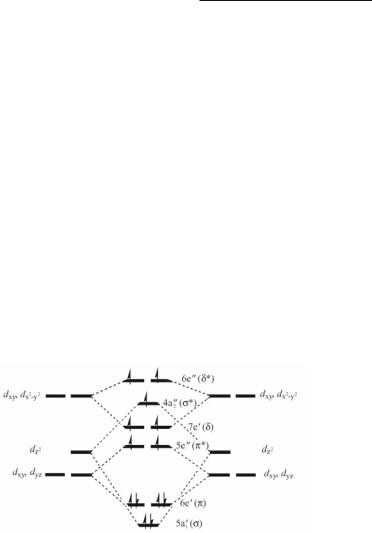
Another remarkable characteristic of these compounds is their magnetism. At room temperature, the µeff values for the formamidinate and benzamidinate compounds are 7.81 and 7.53 BM, respectively, indicating the presence of seven unpaired electrons for each molecule. An EPR spectrum of Fe2(DPhF)3 in a frozen toluene glass gives two signals corresponding to g values of 1.99 and 7.94. If axial symmetry is assumed, the spectrum is consistent with an S value of 7/2. This unusual value for a small dinuclear molecule containing a metal of the first transition series not only is consistent with the bulk magnetic measurements but also with X_-SW and ab initio with configuration interaction (CI) calculations. These have been carried out for both the regular D3h and the distorted C2v symmetries for the model compound Fe2(HNCHNH)3. For these, the standard orbital ordering common for tetragonal paddlewheel compounds and based on D4h symmetry is no longer valid and that based on D3h is shown in Fig. 11.2. According to the X_-SW calculations the energies of the metal–metal based orbitals μ* and /* (5e'' and 4a2'') resulting from the linear combinations of the dz2 and dxz and dyz, and those of the delta-type bonding and antibonding orbitals (7e' and 6e'') resulting from the linear combination of dxy and dx2-y2, are all within a close energy range of 1 eV. By assuming single occupation of all these closely spaced orbitals, the electronic configuration (a1')2(e')2(e')2- (e'')1(e'')1(a2')1(e')1(e')1(e'')1(e'')1 or μ2/2/2/*1/*1μ*1β1β1β*1β*1 with seven unpaired electrons is obtained. The latter can be abbreviated as μ2/4/*2μ*1β2β*2. The calculated equilibrium Fe–Fe distance in the ground state is 2.27 Å as compared to 2.2318 and 2.198 Å in Fe2(DPhF)3 and Fe2(DPhBz)3, respectively. The Fe–Fe bond order is 1.5.
Fig. 11.2. A schematic electron distribution for trigonal paddlewheel molecules with Fe23+ cores showing the seven unpaired electrons in the closely spaced orbitals. Data are from ref. 3.
By eliminating the use of the reducing agent NaEt3BH for the preparation of Fe2(DPhF)3, a compound having an Fe24+ core is formed with four formamidinate bridges.4 However, the molecular structure is significantly different from those found in other paddlewheel M2(RNXNR)4 compounds. A two-fold axis bisects the Fe–Fe vector and lies between the planes formed by the Fe–Fe–N–C–N rings. In contrast to the other compounds known with this stoichiometry but different metal centers (see for example those of cobalt described in Section 11.3.1), there are significant distortions as shown in Fig. 11.3. Two trans bridges are pulled towards one end of the molecule while the other opposite pair are pulled in the opposite direction. The core symmetry is thus reduced from the frequently encountered D4h to D2d symmetry. There is also significant asymmetry in the Fe–N distances; two are short (c. 2.00 Å) and two are long (c. 2.17 Å). The inter-iron separation of 2.462(2) Å 5 is c. 0.26 Å longer than those in Fe2(amidinate)3, discussed above, and only slightly shorter than that in the non-metal–metal

450Multiple Bonds Between Metal Atoms Chapter 11
bonded, formamidinate compound Ni2(DTolF)4 which is discussed in Section 14.2. This distance is similar to those in metal–metal bonded complexes having the heavier congener Ru as shown in Chapter 9. Since this molecule is so distorted and the iron–iron separation is long, it is difficult to decide if a metal–metal bond exists. No theoretical calculations have been done on this molecule. Interestingly, a similar reaction with the benzamidinate analog gave a dinuclear molecule with similar stoichiometry but with two bridging and two chelating benzamidinate ligands. The iron–iron separation of more than 3 Å rules out the possibility of any metal–metal bonding interactions.
Fig. 11.3. The distorted tetragonal paddlewheel molecule in Fe2(DPhF)4.
Several compounds having two iron(II) atoms and carboxylate groups have been made.6,7 Most of them have long Fe···Fe separations which are consistent with the absence of metal–metal bonding. There is a series of tetragonal paddlewheel compounds that have been made with four bulky, bridging carboxylate anions of the type O2CArtol, where O2CArtol is 2,6-di(p-tolyl)benzoate with pyridine-type ligands in axial positions. One compound, Fe2(O2CArtol)4(4-But-py)2, has an Fe···Fe separation of 2.823(1) Å.8 The compound 11.2 undergoes a reversible one-electron oxidation (E1/2 = -0.216 V vs FeCp2+/FeCp2 in CH2Cl2).9 Chemical oxidation with Cp2FePF6 or AgCF3SO3 generates dark green solutions containing the [Fe2(O2CArtol)4-(4-But-py)2]+ cation. The pyridine and THF analogs are also known. The derivative [Fe2(O2CArtol)4(4-But-py)2](CF3SO3) has been structurally characterized. The Fe···Fe separation shortens relative to that of the precursor from 2.823(3) Å to 2.713(3) Å. Even though the iron–iron separation shrinks with the increase of charge, it is unlikely that metal–metal bonding is significant in these highly paramagnetic compounds.
11.2
Finally there is a short Fe–Fe distance of 2.371(4) Å in the organometallic compound
{δ2-C(Mes)=NBut}2Fe2{µ-C(Mes)=NBut}2, where Mes = 2,4,6-Me3C6H2.10 This is made ac-

Iron, Cobalt and Iridium Compounds 451
Murillo
cording to the equation below by insertion of the isonitrile ButNC into a C–Fe bond in Fe2Mes4 which also has a relatively short iron–iron separation of 2.617(1) Å.11 This type of compound falls outside the scope of this book and no further discussion will be provided.
11.3 Dicobalt Compounds
There are only a few dinuclear compounds with Co–Co bonds. These are of the classical paddlewheel type with four bridging ligands, and a few which have a trigonal paddlewheel structure. There are also some with unsupported metal–metal bonds.
11.3.1 Tetragonal paddlewheel compounds
The first authentic Co24+ paddlewheel complex that contains a Co–Co single bond is Co2[(p- tol)2N3]4, in which the strong stabilizing effect of a triazenido ligand towards an M24+ unit is used to advantage.12 This compound is prepared by the interaction of anhydrous CoCl2 with [(p-tol)2N3]- in THF at -78 ºC. This moisture-sensitive, diamagnetic complex has been structurally characterized as its bis-toluene solvate and shown to possess a very short Co–Co separation of 2.265(2) Å. A more efficient synthetic procedure for the preparation of the corresponding amidinate complexes appears to be the reaction of CoCl2(amidine)2 and methyllithium that gives highly pure paddlewheel complexes in good yield according to:
CoCl2(amidine)2 + 4LiMe Α Co2(amidinate)4 + 4LiCl + 4CH4
This has been used to make the corresponding diphenylformamidinato and benzamidinato compounds Co2(DPhF)4 and Co2(DPhBz)4.13 These two compounds cannot be made from anhydrous CoCl2 as in the synthesis of Co2(DTolTA)4.
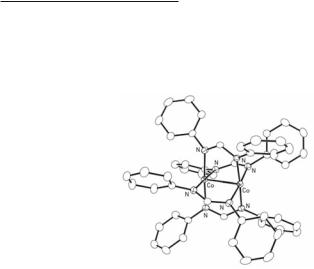
In solution, the red-brown tetra-bridged species are sensitive to the laboratory atmosphere giving solutions with deep blue color containing µ4-oxotetracobalt species but crystals of the tetra-bridged compounds can be handled for a few days in air without noticeable decomposition. The Co–Co single bond distance in the formamidinate compound shown in Fig. 11.4 is 2.3735(7) Å and that of the benzamidinate analog is 2.302(1) Å.14 The torsion angles in these diamagnetic compounds are in the range of 15.5 and 17°. The full pairing of the electrons in these d7–d7 complexes contrasts with the antiferromagnetic bis-quinoline adduct of Co2(O2CPh)4 in which the non-bonded Co···Co separation is more than 2.8 Å.15,16
As shown in Table 11.1, there is a significant increase of the Co–Co distances with a Co24+ core in going from the DTolTA to the DPhBz to the DPhF compound, a pattern similar to that in the corresponding dirhodium compounds (see Chapter 12). Theoretical calculations indicate that these changes are probably due to geometric constraints imposed by the ligands, but other factors, such as the basicity of the ligand set, cannot be ruled out. An early X_-SW calculation17 on the model species Co2(HNNNH)4 failed to predict the expected μ2/4β2β*2/*4 configuration that would be consistent with the diamagnetism of the molecules and a single μ bond between the cobalt atoms. However, later calculations using configuration interaction (CI) methods correctly predict different Co–Co distances for the three known Co2(amidinato)4 compounds. The results of the calculations show that the single bond configuration μ2μ*0 is
452Multiple Bonds Between Metal Atoms Chapter 11
always the leading term in the CI wavefunction of the ground state for each compound. Therefore, it is justified to assign a single μ bond between the metal atoms in all these compounds and an overall electronic configuration of μ2/4β2β*2/*4.
Fig. 11.4. The structure of Co2(DPhF)4. The molecule resides on a crystallographic two-fold axis that passes through the midpoint of the Co–Co single bond.
Table 11.1. Structural data for dicobalt compounds
Compound |
r(Co–Co)a (Å) |
core |
ref. |
Co2(DTolTA)4·2C6H5Me |
2.265(2) |
Co24+ |
12 |
Co2(DPhBz)4 |
2.302(1) |
Co24+ |
14 |
Co2(DPhF)4 |
2.374(1) |
Co24+ |
13 |
[Co2(DPhBz)4]PF6·2.4CH2Cl2b |
2.322(2) |
Co25+ |
13 |
|
2.332(2) |
|
|
Co2(DPhF)3 |
2.385(1) |
Co23+ |
18,19 |
Co2(DPhBz)3 |
2.320(1) |
Co23+ |
19 |
Ba3[Co2(CN)10]·13H2Oc |
2.798(2) |
Co24+ |
20,21 |
|
2.794(2) |
|
|
[Co2(CNCH3)10](ClO4)4 |
2.74(1) |
Co24+ |
22 |
a Distances are given with up to 3 decimal digits. b Two independent molecules.
c Two independent determinations.
Electrochemical studies of Co2(DPhBz)4 in CH2Cl2 solution reveal the existence of two reversible one-electron oxidation waves (E1/2 of 0.29 and 1.45 V vs SCE) and one quasi-reversible reduction which has been assigned to a Co23+ species. Bulk controlled-potential electrolysis of Co2(DPhBz)4 at 0.50 V in CH2Cl2 using Bu4NPF6 as electrolyte revealed that one electron per molecule is involved in the first oxidation. The EPR spectrum of an electrochemically generated (but not fully characterized) reduced species formulated as containing the anion [Co2(DPhBz)4]− gives an axial signal with g of 2.26 and g of 2.01. The g is split into 15 equally spaced lines.
An EPR spectrum of the oxidized [Co2(DPhBz)4]+ cationic species shows a signal at g = 1.98 (g3) split into 15 equally spaced lines by the two 59Co ions (I = 7/2, 100% abundance). The g , or possibly the g1 and g2 signals, is complex and overlaps with a portion of the g3 signal. The splitting of the g3 is consistent with the odd-electron spin density being localized on both cobalt atoms. The oxidized form has been crystallographically characterized in

Iron, Cobalt and Iridium Compounds 453
Murillo
[Co2(DPhBz)4]PF6·2.4CH2Cl2. Two independent molecules in the crystal give metal–metal separations of 2.322(2) and 2.332(2) Å which are slightly longer than those in the neutral molecule (2.302(1) Å). This is of course counter-intuitive if one thinks that the elimination of an electron in an antibonding orbital should increase the bond order from 1 to 1.5. Theoretical calculations showed that upon oxidation the ground state is 2B1u and the μ2β*1configuration is the dominant configuration in the CI wavefunction. Because the electron is being removed from a β* orbital, it has a negligible effect on the length of the metal–metal bond and the intermetallic repulsion due to the increase on the charge in the metal atoms appears to dominate.
11.3.2 Trigonal paddlewheel compounds
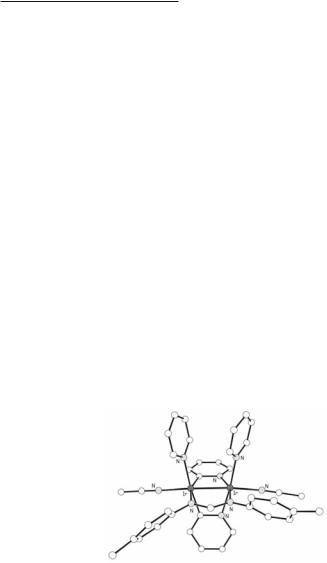
Compounds with Co23+ cores have been crystallographically characterized also but the structures do not correspond to the proposed tetra-bridged [Co2(DPhBz)4]− anion mentioned above. Instead these are similar to those of iron discussed in Section 11.2. There are only three bridging amidinato ligands spanning the dicobalt core which gives a trigonal paddlewheel or trigonal lantern structure. The first such compound Co2(DPhF)3, shown in Fig. 11.5, is prepared in low yield by reaction of CoCl2(HDPhF)2 and BunLi.18 The yield is improved to 63% by addition of the reducing agent NaEt3BH before adding butyllithium:19
2CoCl2(HDPhF)2 + NaEt3BH |
4LiBu |
Co2(DPhF)3 |
+ 3LiCl + NaCl + LiDPhF + ½H2 |
+ BEt3 + 3BuH |
|
In this manner a benzamidinate analog has also been prepared.
Fig. 11.5. The structure of Co2(DPhF)3 showing the idealized D3h symmetry.
The Co–Co distances are 2.385(1) Å for Co2(DPhF)3 and 2.3201(9) Å for Co2(DPhBz)3. The core of the molecules comes very close to having D3h symmmetry. The three N–Co–Co–N torsion angles have an average of only c. 4º and the dihedral angles between ligand planes lie in the range of 115 to 127º. It should be noted that in Fe2(DPhF)3 there is a clear deviation from three-fold symmetry but this was attributed to packing forces (see Section 11.2). The corresponding cobalt analog is not isostructural and the molecules pack in such a way that no marked distortion is engendered. The room temperature magnetic susceptibilities of the compounds with the Co23+ cores are consistent with an electronic ground state having S = 3/2 with a very low-lying S = 5/2 state.
The large difference in the M–M bond lengths in the M2(amidinato)3 compounds with Fe–Fe distances of 2.232(1) and 2.198(2) Å and Co–Co distances of 2.385(1) and 2.320(1) Å for the formamidinato and benzamidinato derivatives is quite remarkable. A study of the electronic structure of the iron compounds (see Section 11.2) leads to the expectation that the two additional electrons in the cobalt analog should occupy /* orbitals which then become fully

454Multiple Bonds Between Metal Atoms Chapter 11
occupied (Fig. 11.2), leading to an electronic configuration μ2/2/2/*2/*2μ*1β1β1β*1β*1. As shown schematically in Fig. 11.6, the μ* (4a'') orbitals, the doubly degenerate β orbitals, (7e'), and the corresponding β* antibonding orbitals are all singly occupied. Thus, there are five unpaired electrons in these Co2(amidinate)3 compounds, and a bond order of 0.5
Fig. 11.6. A schematic electron distribution for trigonal paddlewheel molecules with Co23+ cores. Data are from ref. 3.
11.3.3 Dicobalt compounds with unsupported bonds
An air-stable ruby-red compound of the dinuclear anion [Co2(CN)10]6− has been prepared and characterized as the barium salt Ba3[Co2(CN)10]·13H2O. The crystal structure, done by two independent research groups, is shown in Fig. 11.7. The Co24+ unit lies on a crystallographic two-fold axis which bisects the Co–Co bond of 2.798(2) Å according to one group20 or 2.794(2) Å in the other determination.21 The four equatorial groups are tilted slightly towards the Co–Co unit. The [Co(CN)5]3− groups are rotated 4.5° relative to one another about the Co–Co bond from an ideal D4d geometry. The five independent Co–N bond lengths are equal within experimental error and average 2.151(4) Å. The barium cations, [Co2(CN)10]6− anions, and several water molecules are linked by several types of coordination bridges to give a very tight and cross-linked three-dimensional array which presumably explains the unusual stability towards oxidation.
Fig. 11.7. The [Co2(CN)10]6− anion with an unsupported Co–Co bond.

Iron, Cobalt and Iridium Compounds 455
Murillo
A similar compound, [Co2(CNCH3)10](ClO4)4, contains stronger /-acceptor ligands and [Co2(CNCH3)10]4+ cations.22 In the solid state this is red and diamagnetic. The monomer [Co(CNCH3)5](ClO4)2 has also been isolated; this is green and paramagnetic. The two forms are present in solution. The Co–Co distance in the dimer is 2.74(1) Å. Perhaps the best known compound with an unsupported Co–Co bond is Co2(CO)8. Because these compounds contain /-donor ligands, they fall out of the scope of this book and no further discussion will be provided.
11.3.4 Compounds with chains of cobalt atoms
Many compounds are known to contain extended metal atom chains of three and five Co atoms in which metal–metal bonding exists. These are presented in Chapter 15.
11.4 Di-iridium Compounds
Dinuclear paddlewheel-type complexes with elements of the third transition series from tungsten to platinum are relatively well-known, an exception being iridium. These complexes typically have two metal atoms linked by four bridging ligands; some have axial ligands also. Depending on the electronic configuration of the metal atoms and the type of ligands, metal–metal bond orders can vary from 0.5 to 4. For the lighter congener rhodium (Chapter 12), there are many compounds containing a Rh24+ core with four monoanionic bridging ligands and a single metal–metal bond consistent with a μ2/4β2β*2/*4 electronic configuration.
For iridium the number of such compounds is far smaller. Earlier work provided the first examples of metal–metal bonded compounds of other than the paddlewheel types. The diiridium(I) compound (Ph3P)(CO)Ir(µ-PPh2)2Ir(CO)(PPh3)23,24 was the first one to be formulated as containing an Ir–Ir double bond on the basis of the short Ir–Ir bond distance (c. 2.55 Å) and adherence to the EAN rule. However, this bond-order, bond-length correlation remains suspect in view of the propensity of µ-PR2 ligands to favor short metal–metal contacts. Subsequently, other di-iridium compounds that are believed to contain Ir–Ir multiple bonds have been prepared and characterized, but few possess structures of the L4MML4 or L5MML5 types. In this chapter we consider only the latter type and some closely related species. Others, mainly organometallic compounds, are not considered in detail as they remain outside of the main thrust of this monograph.
11.4.1 Paddlewheel compounds and related species
The one example of a di-iridium complex with an Ir24+ core and four identical monoanionic bridging ligands is the green formamidinate derivative Ir2(DTolF)4, which is prepared25 in small yields by the reaction between (COD)Ir(µ-DTolF)2Ir(O2CCF3)2(H2O), where DTolF = [(p-tolN)2CH]− and COD = 1,5-cyclo-octadiene, and 2 equiv of HDTolF in toluene. It is isostructural with its dirhodium analog (see Section 12.3.3) and has an Ir–Ir distance of 2.524(3) Å as shown in Table 11.2.
Table 11.2. Structural data for di-iridium compounds
Compounda |
r(Ir–Ir)a (Å) r(Ir–L)b (Å) core ref. |
|||
Ir2(DTolF)4 |
2.524(3) |
|
Ir24+ |
25 |
[Ir2(µ-NC5H4)2(µ-DTolF)2(py)2(CH3CN)2]BPh4·2CH3CN |
2.518(1) |
2.13[1] |
Ir24+ |
27 |
[Ir2(µ-DTolF)2(CH3CN)6](BF4)2 |
2.601(1) |
2.18[2] |
Ir24+ |
28 |
[Ir2(µ-DAniF)2(CH3CN)6](BF4)2·2CH3CN |
2.602(1) |
2.209(5) |
Ir24+ |
29 |
Ir2(µ-DAniF)4(δ1-O2CCF3)·2CH2Cl2 |
2.507(1) |
2.139(8) |
Ir25+ |
29 |
456Multiple Bonds Between Metal Atoms Chapter 11
Compounda |
r(Ir–Ir)a (Å) r(Ir–L)b (Å) core ref. |
|||
Ir2(µ-DClPhF)4(δ1-O2CCF3)·2CH2Cl2 |
2.513(1) |
2.16(2) |
Ir25+ |
29 |
Ir2(hpp)4Cl2 |
2.495(1) |
2.643[6] |
Ir26+ |
30 |
Ir2(pc2-)2(py)2 |
2.707(1) |
2.32[2] |
Ir24+ |
32 |
Ir2(tfepma)2Cl2(CH3CN)2 |
2.753(1) |
2.433[1] |
Ir24+ |
31 |
a Distances are given with up to 3 decimal digits.
bIn some cases the average Ir–L bond lengths are quoted. In these instances the estimated derivation, which is given in square brackets, is calculated as [ ] = [−n¨i2/n/(n − 1)]1/2, in which ¨i is the derivation of the ith of n values from the arithmetic mean of the set.
This chemistry was developed following the discovery26 that the reaction between the diiridium(I) compound Ir2(µ-DTolF)2(COD)2 and 2 equiv of AgO2CCF3 produces the unusual complex (COD)Ir(µ-DTolF)2Ir(O2CCF3)2(H2O) in which there is an IrIΑIrIII dative bond. The IrIII center contains two monodentate trifluoroacetate ligands, and an axial water molecule that can be displaced easily by other donor ligands (e.g., DMSO, py, and CH3CN). The Ir–Ir distance in the dark-red pyridine adduct is quite short (2.774(1) Å). The reaction that produces (COD)Ir(µ-DTolF)2Ir(O2CCF3)2(py) can also give the complex [Ir2(µ-NC5H4)2- (µ-DTolF)(py)4]O2CCF3·py, which results from orthometalation of two pyridine ligands.27 A metathesis reaction of this complex with NaBPh4 in acetonitrile has been used to prepare [Ir2(µ-NC5H4)2(µ-DTolF)(py)2(NCCH3)2]BPh4·2CH3CN in which two acetonitrile ligands occupy the axial sites. The Ir–Ir distance in the latter compound is 2.518(1) Å. The X-ray crystal structure determination of this di-iridium species (Fig. 11.8) was not able to distinguish between a head-to-head and head-to-tail arrangement of the two orthometalated pyridine ligands.
Fig. 11.8. The [Ir2(µ-NC5H4)2(µ-DTolF)(py)2(NCCH3)2]+ cation. |
|
|
|
|
When the mixed-valent compound with |
the IrIΑIrIII |
dative bond is |
allowed |
to |
react with (Et3O)BF4 in acetonitrile, the |
compound |
[cis-Ir2(DTolF)2(MeCN)6](BF4)2 |
||
forms.28 Since the number of bridging ligands is only |
two, the Ir–Ir |
distance |
of |
|
2.601(1) Å is longer than the distance in compounds with four bridging ligands. The cyclic voltammogram reveals an irreversible reduction and a reversible oxidation wave with the E1/2 of the latter at +0.77 V vs Ag/AgCl. Analogs containing N,N'-di-p-anisylformamidinato (DAniF) and N,N'-di-p-chlorophenylformamidinato (DClPhF) ligands have been made.29 The former has been characterized by X-ray crystallography and has an Ir–Ir distance of 2.6019(4) Å (see Table 11.2).
In the presence of trifluoroacetate anions, these Ir24+ species react with chlorinated solvents such as CH2Cl2 to give compounds of the type Ir2(µ-DArF)4(δ1-O2CCF3). The metal–metal distances of these Ir25+ compounds are 2.507(1) and 2.513(1) Å for the DAniF and DClPhF
