
Fitts D.D. - Principles of Quantum Mechanics[c] As Applied to Chemistry and Chemical Physics (1999)(en)
.pdf

7.4 Spin±orbit interaction |
203 |
For a hydrogen atom, the potential energy V (r) is given by equation (6.13)
with Z 1 and |
^ |
|
|
|
|
|
|
|
|
Hso becomes |
^ |
|
^ |
: ^ |
|
|
(7:31) |
||
|
|
|
|
|
|
||||
where |
|
|
Hso |
î(r)L |
S |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
î(r) |
|
e2 |
|
|
|
(7:32) |
|
|
|
8ðå0 me2 c2 r3 |
|
|
|||||
|
|
|
|
|
^ |
|
|
|
|
Thus, the total Hamiltonian operator H for a hydrogen atom including spin± |
|||||||||
orbit coupling is |
|
|
|
|
|
|
|
|
|
|
^ |
^ |
^ |
|
^ |
^ |
: ^ |
(7:33) |
|
|
H H0 |
Hso |
H0 |
î(r)L |
S |
||||
where |
^ |
is the Hamiltonian operator for the hydrogen atom without the |
H0 |
inclusion of spin, as given in equation (6.14).
The effect of the spin±orbit interaction term on the total energy is easily shown to be small. The angular momenta jLj and jSj are each on the order of " and the distance r is of the order of the radius a0 of the ®rst Bohr orbit. If we also neglect the small difference between the electronic mass me and the reduced mass ì, the spin±orbit energy is of the order of
8ðåe2"22 2 3 á2jE1j 0 me c a0
where jE1j is the ground-state energy for the hydrogen atom with Hamiltonian
operator |
^ |
as given by equation (6.57) and á is the ®ne structure constant, |
||||||||
H0 |
||||||||||
de®ned by |
|
|
|
|
|
|
|
|
|
|
|
|
á |
e2 |
" |
|
1 |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
4ðå0 |
"c |
me ca0 |
137:036 |
|||||
Thus, the spin±orbit interaction energy is about 5 3 10ÿ5 times smaller than
jE1j. |
|
|
|
|
|
^ |
|
for the hydrogen atom in the absence of |
||||||||
While the Hamiltonian operator H0 |
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
^ |
|
|
^ |
|
|
|
the spin±orbit coupling term commutes with L and with |
S, the total Hamilto- |
|||||||||||||||
nian |
operator |
^ |
in |
equation (7.33) |
does not commute with either |
^ |
^ |
|||||||||
H |
L or |
S |
||||||||||||||
|
|
|
|
|
|
|
|
|
|
^ |
: ^ |
|
|
|
|
|
because of the presence of the scalar product L |
S. To illustrate this feature, |
|||||||||||||||
|
|
|
|
|
|
^ |
^ |
: |
^ |
^ |
^ |
: ^ |
|
|
|
|
we consider the commutators [Lz |
, L |
|
S] and [Sz, L |
S], |
|
|
|
|
||||||||
^ |
^ |
: ^ |
^ |
^ |
^ |
^ ^ |
^ |
^ |
^ ^ |
^ |
^ ^ |
^ |
|
|
||
[Lz, |
L |
S] |
[Lz, (Lx Sx Ly S y |
Lz Sz)] [Lz, Lx]Sx |
[Lz, Ly]S y 0 |
|
||||||||||
|
|
|
^ |
^ |
^ |
^ |
|
|
|
|
|
|
|
|
(7:34) |
|
|
|
i"(Ly Sx ÿ Lx S y) 60 |
|
|
|
|
|
|
|
|||||||
^ |
^ |
: ^ |
^ ^ |
^ |
^ ^ |
^ |
|
^ |
^ |
|
^ ^ |
60 |
|
(7:35) |
||
[Sz, |
L |
S] |
[Sz, |
Sx]Lx [Sz, S y]Ly |
i"(Lx S y |
ÿ Ly Sx) |
|
|||||||||
where equations (5.10) and (7.2) have been used. Similar expressions apply to
the other components of |
^ |
^ |
L and |
S. Thus, the vectors L and S are no longer |
7.4 Spin±orbit interaction |
205 |
motion, but S2 is. Since J is ®xed in direction and magnitude, both J and J2
are constants of motion. |
|
|
|
|
|
|
|
|
|
|||||||||||
If we form the cross product |
^ |
^ |
|
|
|
|
|
|
||||||||||||
J 3 J and substitute equations (7.36), (5.11), |
||||||||||||||||||||
and (7.3), we obtain |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
^ ^ |
^ |
|
|
^ |
|
|
^ |
^ |
|
^ ^ |
^ ^ |
|
^ |
^ |
^ |
||||
|
J 3 J (L S) 3 (L S) |
(L 3 L) (S 3 S) |
i"L |
i"S |
i"J |
|||||||||||||||
where |
the |
cross |
|
terms |
^ |
^ |
|
^ |
^ |
each |
other. Thus, the |
|||||||||
|
(L |
3 S) |
and (S 3 L) cancel |
|||||||||||||||||
|
|
^ |
obeys equation (5.12) and the quantum-mechanical treatment of |
|||||||||||||||||
operator J |
||||||||||||||||||||
Section 5.2 applies to the total angular momentum. Since |
^ |
^ |
^ |
|||||||||||||||||
Jx, J y, and Jz each |
||||||||||||||||||||
|
|
|
^2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
^ |
|
commute with J |
|
but do not commute with one another, we select Jz and seek |
||||||||||||||||||
the |
simultaneous eigenfunctions |
j |
nlsjm |
ji |
of the set of mutually commuting |
|||||||||||||||
|
^ |
2 |
S |
2 |
, |
J |
2 |
, and |
^ |
|
|
|
|
|
|
|||||
operators H, L , |
|
|
Jz |
|
|
|
|
|
|
|
|
|||||||||
|
^ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(7:37a) |
|
Hjnlsjmji Enjnlsjmji |
|
|
|
|
|
|
|
||||||||||||
|
^2 |
jnlsjmji l(l |
2 |
jnlsjmji |
|
|
|
|
|
(7:37b) |
||||||||||
|
L |
1)" |
|
|
|
|
|
|||||||||||||
|
^2 |
jnlsjmji s(s |
2 |
|
|
|
|
|
|
|
(7:37c) |
|||||||||
|
S |
1)" jnlsjmji |
|
|
|
|
|
|||||||||||||
|
^2 |
jnlsjmji j(j |
2 |
|
|
|
|
|
|
|
(7:37d) |
|||||||||
|
J |
1)" jnlsjmji |
|
|
|
|
|
|||||||||||||
|
^ |
|
|
|
|
|
|
|
|
|
|
|
|
mj ÿj, ÿj 1, . . . , |
j |
ÿ 1, j |
(7:37e) |
|||
|
Jzjnlsjmji mj"jnlsjmji, |
|
||||||||||||||||||
From the expression |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
^ |
|
|
|
|
|
|
|
^ |
^ |
|
|
|
|
|
|
|
|
|
|
|
Jzjnlsjmji (Lz Sz)jnlsjmji (m ms)"jnlsjmji |
|
||||||||||||||||
obtained from (7.36), (5.28b), and (7.5), we see that |
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
mj m ms |
|
|
|
(7:38) |
||||
The quantum number j takes on the values |
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
l s, l s ÿ 1, l s ÿ 2, . . . , jl ÿ sj |
|
|
|
|||||||||
The argument leading to this conclusion is somewhat complicated and may be found elsewhere.3 In the application being considered here, the spin s equals 12
and the quantum number j can have only two values |
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
j l 21 |
|
|
|
|
(7:39) |
||
The resulting vectors J are shown in Figure 7.2. |
|
|
|
|
|
||||||||||
The scalar product |
^ |
: ^ |
|
|
|
|
|
|
|
|
|||||
L |
|
S in equation (7.33) may be expressed in terms of |
|||||||||||||
|
|
|
|
|
|
|
^ |
|
|
|
|
|
|
|
|
operators that commute with H by |
|
|
|
|
|
|
|
||||||||
^ |
: ^ |
1 ^ |
^ |
: |
|
^ |
^ |
1^ |
: ^ |
1^ : |
^ |
1 ^2 |
^2 |
^2 |
) (7:40) |
L |
S |
2(L S) |
|
(L S) ÿ |
2L |
L ÿ |
2S |
S |
2(J |
ÿ L |
ÿ S |
||||
3B. H. Brandsen and C. J. Joachain (1989) Introduction to Quantum Mechanics (Addison Wesley Longman, Harlow, Essex), pp. 299, 301; R. N. Zare (1988) Angular Momentum (John Wiley & Sons, New York), pp. 45±8.
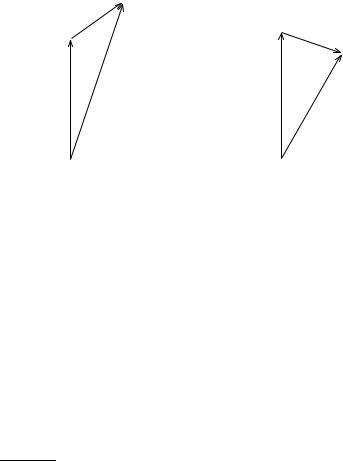
206 |
|
Spin |
|
|
S |
|
|
S |
|
|
J |
|
L |
L |
|
J |
|
|
|
j 5 l 1 |
1 |
j 5 l 2 |
1 |
2 |
2 |
Figure 7.2 The total angular momentum vectors J obtained from the sum of L and S for s 12 and s ÿ12.
|
|
^ |
|
|
|
|
|
|
|
|
|
|
|
so that H becomes |
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
^ |
^ |
1 |
^2 |
^2 |
^2 |
) |
(7:41) |
|
|
|
|
|
|
H |
H0 |
2î(r)(J |
ÿ L |
ÿ S |
|||
Equation (7.37a) then takes the form |
|
|
|
|
|
||||||||
|
^ |
1 |
2 |
î(r)[ j(j |
1) |
ÿ l(l 1) ÿ s(s 1)]gjnlsjmji Enjnlsjmji |
(7:42) |
||||||
or |
fH0 |
2" |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
l"2 |
|
|
|
|
|
|
|
|
||
|
H^ 0 |
|
|
î(r) jn, l, 21, l 21, mji Enjn, l, 21, l 21, mji if j l 21 |
|||||||||
|
|
2 |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
(7:43a) |
H^ |
|
ÿ |
(l 1)"2 |
î(r) |
j |
n, l, |
1, l |
ÿ |
1, m |
ji |
E |
nj |
n, l, |
1, l |
ÿ |
1, m |
ji |
|
0 |
2 |
|
|
ÿ2 |
2 |
|
|
ÿ2 |
2 |
if j l ÿ 12 (7:43b) where equations (7.37b), (7.37c), (7.37d), and (7.39) have also been introduced.
Since the spin±orbit interaction energy is small, the solution of equations (7.43) to obtain En is most easily accomplished by means of perturbation theory, a technique which is presented in Chapter 9. The evaluation of En is left as a problem at the end of Chapter 9.
Problems
7.1Determine the angle between the spin vector S and the z-axis for an electron in spin state jái.
7.2Prove equation (7.19) from equations (7.15) and (7.17).
Problems |
207 |
7.3Show that the pair of operators ó y, ó z anticommute.
7.4Using the Pauli spin matrices in equation (7.25) and the spinors in (7.13),
(a) construct the operators ó and ó corresponding to ^ and ^
ÿ S Sÿ
(b) operate on jái and on jâi with ó 2, óz, ó , óÿ, óx, and ó y and compare the results with equations (7.14), (7.15), (7.16), and (7.17).
7.5Using the Pauli spin matrices in equation (7.25), verify the relationships in (7.19) and (7.22).
8.1 Permutations of identical particles |
209 |
of mass m. If we label one of the particles as particle 1 and the other as particle
|
^ |
|
|
|
|
|
2, then the Hamiltonian operator H(1, 2) for the system is |
|
|||||
^ |
|
p^2 |
|
p^2 |
|
|
|
1 |
|
2 |
|
|
|
H(1, 2) |
2m |
2m V(q1, q2) |
(8:1) |
|||
where qi (i 1, 2) represents |
|
the |
three-dimensional (continuous) |
spatial |
||
coordinates ri and the (discrete) spin coordinate ói of particle i. In order for these two identical particles to be indistinguishable from each other, the Hamiltonian operator must be symmetric with respect to particle interchange, i.e., if the coordinates (both spatial and spin) of the particles are interchanged,
^ (1, 2) must remain invariant
H
|
|
^ |
|
^ |
|
|
H(1, 2) |
H(2, 1) |
|
If |
^ |
^ |
|
then the corresponding SchroÈdinger |
H(1, 2) and |
H(2, 1) were to differ, |
|||
equations and their solutions would also differ and this difference could be used to distinguish between the two particles.
The time-independent SchroÈdinger equation for the two-particle system is
^ |
EíØí(1, 2) |
(8:2) |
H(1, 2)Øí(1, 2) |
where í delineates the various states. The notation Øí(1, 2) indicates that the ®rst particle has coordinates q1 and the second particle has coordinates q2. If we exchange the two particles so that particles 1 and 2 now have coordinates q2 and q1, respectively, then the SchroÈdinger equation (8.2) becomes
^ |
|
^ |
(1, 2)Øí(2, 1) |
EíØí(2, 1) |
(8:3) |
H(2, 1)Øí(2, 1) |
H |
||||
where we have noted that |
^ |
|
|
|
|
H(1, 2) is symmetric. Equation (8.3) shows that |
|||||
Ø (2, 1) is also an eigenfunction of ^ (1, 2) belonging to the same eigenvalue
í H
Eí. Thus, any linear combination of Øí(1, 2) and Øí(2, 1) is also an eigen-
function of ^ (1, 2) with eigenvalue . For simplicity of notation in the
H Eí
following presentation, we omit the index í when it is clear that we are referring to a single quantum state.
The eigenfunction Ø(1, 2) has the form of a wave in six-dimensional space. The quantity Ø (1, 2)Ø(1, 2) dr1 dr2 is the probability that particle 1 with spin function ÷1 is in the volume element dr1 centered at r1 and simultaneously particle 2 with spin function ÷2 is in the volume element dr2 at r2. The product Ø (1, 2)Ø(1, 2) is, then, the probability density. The eigenfunction Ø(2, 1) also has the form of a six-dimensional wave. The quantity Ø (2, 1)Ø(2, 1) is the probability density for particle 2 being at r1 with spin function ÷1 and simultaneously particle 1 being at r2 with spin function ÷2. In general, the two eigenfunctions Ø(1, 2) and Ø(2, 1) are not identical. As an example, if Ø(1, 2) is
210 |
Systems of identical particles |
||
Ø(1, |
2) eÿar1 eÿbr2 (br2 ÿ 1) |
||
where r1 jr1j and r2 jr2j, then Ø(2, 1) would be |
|||
Ø(2, 1) |
|
eÿar2 eÿbr1 (br |
1) Ø(1, 2) |
|
1 ÿ |
6 |
|
Thus, the probability density of the pair of particles depends on how we label the two particles. Since the two particles are indistinguishable, we conclude that neither Ø(1, 2) nor Ø(2, 1) are desirable wave functions. We seek a wave function that does not make a distinction between the two particles and, therefore, does not designate which particle is at r1 and which is at r2.
To that end, we now introduce the linear hermitian |
|
^ |
||||||
exchange operator P, |
||||||||
which has the property |
|
|
|
|
|
|
||
|
|
^ |
(1, 2) f (2, 1) |
|
(8:4) |
|||
|
|
P f |
^ |
|||||
where f (1, 2) |
is an |
arbitrary |
function |
of q1 and |
operates on |
|||
q2. If P |
||||||||
^ |
|
|
|
|
|
|
|
|
H(1, 2)Ø(1, 2), we have |
|
|
|
|
|
|||
^ ^ |
|
^ |
|
|
^ |
^ |
^ |
|
P[H(1, 2)Ø(1, 2)] |
H(2, 1)Ø(2, 1) |
H(1, 2)Ø(2, 1) H(1, 2)PØ(1, 2) |
||||||
|
|
|
|
|
|
|
(8:5) |
|
where we have used the fact that |
^ |
|
|
|
||||
H(1, 2) is symmetric. From equation (8.5) we |
||||||||
^ |
^ |
|
|
|
|
|
|
|
see that P and |
H(1, 2) commute |
|
|
|
|
|
||
|
|
^ |
|
^ |
|
|
(8:6) |
|
|
|
[P, |
H(1, 2)] 0, |
|
||||
|
|
^ |
|
^ |
|
|
|
|
Consequently, the operators P and |
H(1, 2) have simultaneous eigenfunctions. |
|||||||
|
|
|
|
^ |
|
|
|
|
|
If Ö(1, 2) is an eigenfunction of P, the corresponding eigenvalue ë is given |
|||||||||
by |
|
|
|
|
|
|
|
|
|
|
|
|
|
^ |
|
|
|
|
(8:7) |
We then have |
PÖ(1, 2) ëÖ(1, 2) |
|
|
||||||
|
|
|
|
|
|
|
|||
^2 |
Ö(1, 2) |
^ ^ |
|
^ |
|
^ |
|
2 |
(8:8) |
P |
P[PÖ(1, 2)] |
P[ëÖ(1, 2)] ëPÖ(1, 2) ë Ö(1, 2) |
|||||||
Moreover, operating on Ö(1, 2) twice in succession by |
^ |
returns the |
two |
||||||
P |
|||||||||
particles to their original order, so that |
|
|
|
|
|
||||
|
|
^2 |
|
^ |
Ö(1, 2) |
|
|
(8:9) |
|
|
|
P |
Ö(1, 2) PÖ(2, 1) |
|
|
||||
|
|
|
|
^ |
2 |
|
2 |
|
^ |
From equations (8.8) and (8.9), we see that P |
|
1 and that ë |
1. Since P is |
||||||
hermitian, the eigenvalue ë is real and we obtain ë 1. |
|
|
|
||||||
There are |
only two functions which are simultaneous |
eigenfunctions of |
|||||||
^ |
|
^ |
|
|
|
|
|
|
|
H(1, 2) and |
P with respective eigenvalues E and 1. These functions are the |
||||||||
combinations |
|
|
|
|
|
|
|
||
|
|
ØS 2ÿ1=2[Ø(1, 2) Ø(2, 1)] |
|
(8:10a) |
|||||
|
|
ØA 2ÿ1=2[Ø(1, 2) ÿ Ø(2, 1)] |
|
(8:10b) |
|||||
which satisfy the relations |
|
|
|
|
|
|
|||
8.1 Permutations of identical particles |
211 |
^ |
(8:11a) |
PØS ØS |
|
^ |
(8:11b) |
PØA ÿØA |
The factor 2ÿ1=2 in equations (8.10) normalizes ØS and ØA if Ø(1, 2) is normalized. The combination ØS is symmetric with respect to particle interchange because it remains unchanged when the two particles are exchanged. The function ØA, on the other hand, is antisymmetric with respect to particle interchange because it changes sign, but is otherwise unchanged, when the particles are exchanged.
The functions ØA and ØS are orthogonal. To demonstrate this property, we note that the integral over all space of a function of two or more variables must
be independent of the labeling of those variables |
|
|
… |
… f (x1, . . . , xN ) dx1 . . . dxN … … f (y1, . . . , yN ) dy1 . . . d yN |
(8:12) |
In particular, we have |
|
|
or |
…… f (1, 2) dq1 dq2 …… f (2, 1) dq1 dq2 |
|
hØ(1, 2)jØ(2, 1)i hØ(2, 1)jØ(1, 2)i |
|
|
|
(8:13) |
|
where f (1, 2) Ø (1, 2)Ø(2, 1). Application of equation (8.13) to hØS jØAi gives
^ ^ |
(8:14) |
hØS jØAi hPØS jPØAi |
Applying equations (8.11) to the right-hand side of (8.14), we obtain hØS jØAi ÿhØS jØAi
Thus, the scalar product hØS jØAi must vanish, showing that ØA and ØS are orthogonal.
If the wave function for the system is initially symmetric (antisymmetric), then it remains symmetric (antisymmetric) as time progresses. This property
follows from the time-dependent SchroÈdinger equation |
|
|||
|
@Ø(1, 2) |
|
^ |
|
i" |
|
|
H(1, 2)Ø(1, 2) |
(8:15) |
@t |
||||
Since ^ (1, 2) is symmetric, the time derivative @Ø=@ has the same symmetry
H t
as Ø. During a small time interval Ät, therefore, the symmetry of Ø does not change. By repetition of this argument, the symmetry remains the same over a succession of small time intervals, and by extension over all time.
Since ØS does not change and only the sign of ØA changes if particles 1 and 2 are interchanged, the respective probability densities ØS ØS and ØAØA are independent of how the particles are labeled. Neither speci®es which particle
