
Garrett R.H., Grisham C.M. - Biochemistry (1999)(2nd ed.)(en)
.pdf


25.1 ● The Fatty Acid Biosynthesis and Degradation Pathways Are Different |
813 |
|
|
|
|
|
|
|
|
|
|
|
AT |
KSase |
MT |
|
|
|
|
|
|
|
AT |
KSase |
|
MT |
|
|
|
|
|
|
AT |
KSase |
MT |
|
|||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
SH |
|
OH |
|
|
|
|
|
|
|
O |
|
SH |
|
O |
|
|
|
|
|
|
OH |
|
SH |
O |
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
O |
1 |
O |
|
C |
|
|
|
|
C |
|
|
|
O |
|
2 |
|
|
|
|
|
|
|
C |
|
O |
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H3C |
|
|
O |
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3 |
|
CH2 |
|
|
|
|
|
CH2 |
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
CH3C |
|
|
SCoA |
|
|
O2CCH2C |
|
|
SCoA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO–2 |
|
|
|
|
|
|
|
|
|
CO–2 |
|
||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
SH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SH |
|
|
|
|
|
|
S |
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ACP |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ACP |
|
|
|
|
|
|
|
|
|
|
|
|
ACP |
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
AT KSase |
MT |
|
AT |
|
KSase |
MT |
|
|
|
|
|
|
|
AT KSase |
MT |
|
|
AT |
KSase |
MT |
|
||||||||||||||||||||||||||||||||||
|
|
HS |
|
|
|
|
|
|
|
|
|
HS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
CH3 |
|
|
|
CO2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
HO |
OH |
HO |
OH |
|
|
|
|
|
OH |
S |
OH |
|
|
OH |
|
S |
O |
|
|||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
6 |
|
|
|
|
|
|
C |
|
O |
|
5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
– |
O |
|
|
|
C |
|
O |
|
|
|
|
O |
|
C |
|
C |
|
O |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3 |
|
|
|
|
|
|
|
|
|
CH3 |
|
CH2 |
|
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
C |
|
O |
|
|
|
|
|
|
|
C |
|
O |
|
|
|
O |
|
|
C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO–2 |
|
|||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
S |
|
|
|
|
|
|
|
|
|
|
|
|
|
S |
|
|
|
|
|
|
|
|
|
|
S |
|
|
|
|
|
|
|
|
|
SH |
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
ACP |
|
|
|
|
|
|
|
|
|
|
|
|
ACP |
|
|
|
|
|
|
|
|
|
|
|
|
ACP |
|
|
|
|
|
|
|
|
ACP |
|
|
|
|
||||||||||||||
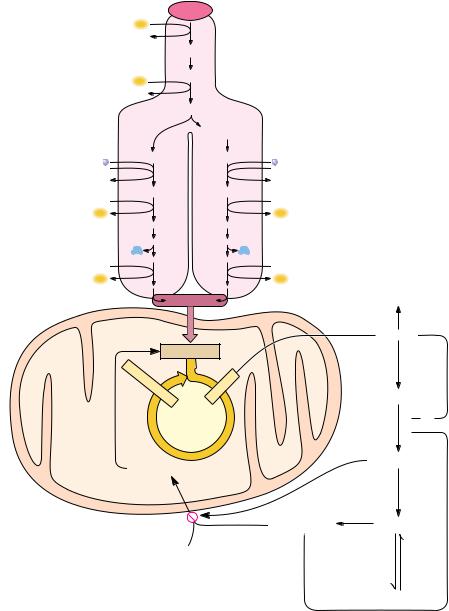
FIGURE 25.11 ● The mechanism of the fatty acyl synthase reaction in eukaryotes.
(1) Acetyl and malonyl groups are loaded onto acetyl transferase and malonyl transferase, respectively. (2) The acetate unit that forms the base of the nascent chain is transferred first to the acyl carrier protein domain and (3) then to the -ketoacyl synthase. (4) Attack by ACP on the carbonyl carbon of a malonyl unit on malonyl transferase forms malonylACP. (5) Decarboxylation leaves a reactive, transient carbanion that can attack the carbonyl carbon of the acetyl group on the -ketoacyl synthase. (6) Reduction of the keto group, dehydration, and saturation of the resulting double bond follow, leaving an acyl group on ACP, and steps 3 through 6 repeat to lengthen the nascent chain.
resemble the reverse of the reactions of fatty acid oxidation (and the conversion of succinate to oxaloacetate in the TCA cycle). This synthetic cycle now repeats until the growing chain is 16 carbons long. It is then released by the thioesterase domain on the synthase. The amino acid sequence of the thioesterase domain is homologous with serine proteases; the enzyme has an active-site serine that carries out nucleophilic attack on the carbonyl carbon of the fatty acyl thioester to be cleaved.
Further Processing of C16 Fatty Acids
Additional Elongation
As seen already, palmitate is the primary product of the fatty acid synthase. Cells synthesize many other fatty acids. Shorter chains are easily made if the chain is released before reaching 16 carbons in length. Longer chains are made through special elongation reactions, which occur both in the mitochondria and at the surface of the endoplasmic reticulum. The ER reactions are actually quite similar to those we have just discussed: addition of two-carbon units

816 Chapter 25 ● Lipid Biosynthesis
The Unsaturation Reaction May Be Followed by Chain Elongation
Additional chain elongation can occur following this single desaturation reaction. The oleoyl-CoA produced can be elongated by two carbons to form a 20:1 cis- 11 fatty acyl-CoA. If the starting fatty acid is palmitate, reactions similar to the preceding scheme yield palmitoleoyl-CoA (16:1 cis- 9), which subsequently can be elongated to yield cis-vaccenic acid (18:1 cis- 11). Similarly, C16 and C18 fatty acids can be elongated to yield C22 and C24 fatty acids, such as are often found in sphingolipids.
Biosynthesis of Polyunsaturated Fatty Acids
Organisms differ with respect to formation, processing, and utilization of polyunsaturated fatty acids. E. coli, for example, does not have any polyunsaturated fatty acids. Eukaryotes do synthesize a variety of polyunsaturated fatty acids, certain organisms more than others. For example, plants manufacture double bonds between the 9 and the methyl end of the chain, but mammals cannot. Plants readily desaturate oleic acid at the 12-position (to give linoleic acid) or at both the 12and 15-positions (producing linolenic acid). Mammals require polyunsaturated fatty acids, but must acquire them in their diet. As such, they are referred to as essential fatty acids. On the other hand, mammals can introduce double bonds between the double bond at the 8- or 9-posi- tion and the carboxyl group. Enzyme complexes in the endoplasmic reticulum desaturate the 5-position, provided a double bond exists at the 8-position, and form a double bond at the 6-position if one already exists at the 9-position. Thus, oleate can be unsaturated at the 6,7-position to give an 18:2 cis- 6, 9 fatty acid.
Arachidonic Acid Is Synthesized from Linoleic Acid by Mammals
Mammals can add additional double bonds to unsaturated fatty acids in their diets. Their ability to make arachidonic acid from linoleic acid is one example (Figure 25.15). This fatty acid is the precursor for prostaglandins and other biologically active derivatives such as leukotrienes. Synthesis involves formation of a linoleoyl ester of CoA from dietary linoleic acid, followed by introduction of a double bond at the 6-position. The triply unsaturated product is then elongated (by malonyl-CoA with a decarboxylation step) to yield a 20-carbon fatty acid with double bonds at the 8-, 11-, and 14-positions. A second desaturation reaction at the 5-position followed by an acyl-CoA synthetase reaction (Chapter 24) liberates the product, a 20-carbon fatty acid with double bonds at the 5-, 8-, 11-, and 14-positions.
Regulatory Control of Fatty Acid Metabolism—An Interplay of
Allosteric Modifiers and Phosphorylation–Dephosphorylation Cycles
The control and regulation of fatty acid synthesis is intimately related to regulation of fatty acid breakdown, glycolysis, and the TCA cycle. Acetyl-CoA is an important intermediate metabolite in all these processes. In these terms, it is easy to appreciate the interlocking relationships in Figure 25.16. Malonyl-CoA can act to prevent fatty acyl-CoA derivatives from entering the mitochondria by inhibiting the carnitine acyltransferase that is responsible for this transport. In this way, when fatty acid synthesis is turned on (as signaled by higher levels of malonyl-CoA), -oxidation is inhibited. As we pointed out earlier, citrate is an important allosteric activator of acetyl-CoA carboxylase, and fatty acyl-CoAs




 C C S
C C S NADP
NADP CH
CH CH
CH
 2 H
2 H Malate
Malate  Oxaloacetate
Oxaloacetate Citrate
Citrate


 AMP
AMP