
Solid Support Oligosaccharide Synthesis
.pdf
118 SOLID-PHASE OLIGOSACCHARIDE SYNTHESIS
26
phosphinimidates, or phosphorous(V) compounds as glycosyl donors was not extensively studied until 1999. In this chapter we summarize the most recent developments concerning the synthesis and application of glycosyl phosphates as glycosylating agents.
6.2 GLYCOSYL PHOSPHATE DONORS
6.2.1 Synthesis and Reactivity
Ikegami and coworkers were the first to address the potential of glycosyl phosphates as glycosyl donors.23 A series of diphenyl phosphate donors 2 were readily obtained by deprotonating the free reducing sugar with n-butyllithium and subsequent treatment with diphenyl phosphorochloridate (Scheme 6.1A). The 2-O-benzylated glycosyl donors 2 showed remarkable β-selectivity and high yields in coupling reactions with various glycosyl acceptors. The reactions were carried out in propionitrile in the presence of equimolar amounts of TMSOTf as promoter. Acid-sensitive groups such as epoxides and acetals survived the coupling conditions. Corresponding 2-O-benzoyl protected glycosyl diphenyl phosphates 4 exhibited complete β-selectivity with coupling yields up to 95%, including coupling to the hindered C4 position on glucose in over 83% yield.
Although this synthesis preferentially lead to α-phosphates, either anomer could be prepared selectively by direct anomeric phosphorylation of the lactol using diphenyl phosphorochloridate and DMAP choosing thermodynamic or kinetic conditions.27 Glycosyl α- and β-diphenyl and dialkyl phosphate donors have also been synthesized by nucleophilic displacement of an anomeric trichloroacetimidate. Evaluation of α-diaryl phosphate 7 and cyclic β-phosphate 8 as glycosyl donors under promotion by catalytic amounts of BF3 etherate in a nonparticipating solvent required ambient temperature to proceed in reasonable yield, although revealing poor stereoselectivities (Scheme 6.2).28
A |
|
|
|
|
|
|
|
|
|
|
|
BnO |
R |
1) n-BuLi |
|
R |
|
R'OH, TMSOTf, |
|
R |
|||
O |
2) ClP(O)(OPh)2 |
BnO |
O |
|
-78 °C, EtCN |
|
BnO |
O |
|||
BnO |
BnO |
OH |
|
BnO |
O |
|
|
|
BnO |
OR' |
|
|
|
|
BnOO |
P |
OPh |
78-88% |
|
|
BnO |
||
|
|
|
|
|
|
α/β |
|||||
R = CH2OBn, CO2Me |
|
|
|
OPh |
|
|
= 14:86-3:97 |
||||
|
|
2 |
|
|
|
|
|||||
|
1 |
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
||
B |
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
|
R'OH, TMSOTf, |
|
|
|
|
|
||
|
|
BzO |
O |
BnO |
R |
|
|
|
|||
|
|
BzO |
O |
-78 °C, EtCN |
|
O |
OR' |
|
|
||
|
|
BzOO P OPh |
|
|
|
BnO |
|
|
|||
|
|
|
72-95% |
|
|
|
|
||||
|
|
R = CH2OBz, H |
OPh |
|
|
|
BnO |
|
|
|
|
|
|
|
|
|
|
5 |
|
|
|
||
|
|
4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 6.1 Glycosyl phosphates functioning as glycosl donors.

|
|
|
|
|
6.2 GLYCOSYL PHOSPHATE DONORS |
119 |
||||
BnO |
|
|
O |
|
|
|
|
|
|
|
O |
|
HO P OpClPh |
BnO |
|
|
|
|
|
||
BnO |
|
O |
|
|
|
|
||||
|
|
|
BnO |
|
|
|
|
|||
|
|
OpClPh |
|
|
|
|
|
|||
BnO |
|
|
|
O |
|
|
|
|||
BnOO |
|
BnO |
|
|
|
|
||||
|
NH |
BnO |
|
|
|
|||||
|
|
P OpClPh |
|
|
|
|||||
6 |
|
|
|
|
|
O |
|
|
|
|
|
CCl3 |
|
7 |
OpClPh |
|
|
|
|||
|
|
|
|
|
|
|||||
|
|
O |
|
R'OH, cat. BF3·Et2O |
|
|
|
|||
|
|
|
|
|
|
|||||
|
HO P O |
|
|
|
|
|||||
|
|
r. t., 45-81% |
BnO |
|
|
|||||
|
O |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
O |
|
||
|
|
|
|
|
|
|
BnO |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BnO |
BnO |
OR' |
|
|
|
|
|
|
|
|
|
||
BnO |
O |
O |
|
|
|
|
9 |
|
||
BnO |
|
|
|
|
|
|
||||
O P O |
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
||||
BnO |
|
|
|
|
|
|
|
|
|
|
BnO |
O |
|
|
8 |
|
Scheme 6.2 α-Diaryl phosphate and cyclic β-phosphate functioning as glycosyl donors.
When activated by excess or equimolar TMSOTf, glycosyl phosphate donors proved to be very efficient in a few total syntheses of glycosylated compounds. Boger and Honda used a 2-O-acetyl mannosyl phosphate for the high-yielding construction of an α-mannosidic linkage in the total synthesis of bleomycin A.29 α-Fucosidic linkages serve as examples of difficult-to-achieve 1,2-cis-glycosidic linkages, which have been installed employing fucosyl phosphate donors in the synthesis of Shimofuridin analog 13 (Scheme 6.3A)30 and fucosidase substrate 20 (Scheme 6.3B).31 Interestingly, when fucosylations were carried out in the presence of catalytic amounts of promoter, the coupling proceeded with poor stereoselectivity and in considerably lower yield, indicating either an SN2-type mechanism or a neighboring-group participation by the 2-O-acetyl group.
We have disclosed a straightforward one-pot procedure for the synthesis of glycosyl phosphates from glycals. Diastereoselective epoxidation of suitably protected glycals followed by ring-opening addition of a phosphate diester and in situ protection of the generated free C2-hydroxyl gave access to a wide range of differentiated glucosyl-, and galactosyl phosphates in high yields (Scheme 6.4).32 Generally, this procedure yielded the more reactive β-phosphates when carried out in noncoordinating solvents such as dichloromethane or toluene. This selectivity, however, could in most cases be effectively reversed by simply switching the solvent to THF as exemplified in the synthesis of 24. This approach minimizes the number of protecting group manipulations in the preparation of glycosyl phosphates, thus rendering phosphates an attractive alternative to the established donors that often require lengthy syntheses.
β-Phosphates were smoothly activated by equimolar amounts of TMSOTf at –78°C, while α-phosphates reacted readily at –20°C with various carbohydrate acceptors. Employing these conditions, glycosyl phosphates served as powerful glycosyl donors in the β-selective formation of glycosidic linkages with or without a C2 participating group (Scheme 6.5).

120 SOLID-PHASE OLIGOSACCHARIDE SYNTHESIS
A |
|
|
|
O |
SEt |
|
|
|
|
|
|
|
|
|
OBn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AcOOBn 10 |
|
|
|
|
|
|
|
|
|
|
|
NIS, HOP(O)(Bu)2 |
|
|
|
O |
|
|
|
|
|
|
92% |
|
|
|
R |
|
|
|
|
O |
|
O |
|
|
|
N |
|
|
|
|
|
|
|
|
N |
|||
|
|
|
|
R |
|
|
|
|
||
|
|
N |
|
O P OBu |
|
|
|
|
|
|
|
|
|
N |
|
|
|
N N |
|
||
|
|
|
|
OBnOBu |
|
|
|
|
||
|
|
N |
N |
O |
iPr |
O |
O |
|
|
|
|
|
OBn |
11 |
|
|
|||||
|
|
|
|
|
Si |
|
|
|
||
iPr |
O |
O |
|
AcO |
|
iPr |
|
|
|
|
|
Si |
|
|
TMSOTf, 4 Å MS, 53% |
|
O |
O |
O |
|
|
iPr |
|
|
|
|
|
Si |
|
|||
|
O |
O OH |
|
|
|
iPr iPr |
|
13 |
|
|
|
Si |
|
|
|
|
|
|
|
|
|
iPr iPr |
12 |
|
|
|
|
|
O |
OBn |
|
|
R = CH2OC(O)CMe3 |
|
|
|
|
AcOOBn |
|
|
|||
|
|
|
|
|
|
|
|
|||
B |
|
O |
|
|
|
|
|
|
|
AcO |
O |
|
|
|
|
P |
|
O |
|
|
|
O |
|
AcO |
|
|
|
||
|
|
|
|
|
|
|
|
|
AcO |
|
||||
|
|
Cl |
O |
|
|
|
P |
|
AcO |
HOOAc |
|
O |
||
|
|
|
|
|
|
O |
|
|
AcO |
|||||
|
OH |
N-methylimidazole, |
|
O |
O |
|
16 |
|
|
|||||
|
|
|
|
|
|
AcO |
OOAc |
|||||||
|
O OAc |
r.t., 65% |
|
|
|
O |
OAc |
TMSOTf, -78 °C, 63% |
|
|||||
AcO |
OAc |
|
|
|
|
|
AcOOAc |
|
|
|
O |
OAc |
||
|
|
|
|
|
15 |
|
|
|
|
|||||
14 |
|
|
|
|
|
|
|
|
|
AcOOAc |
||||
|
|
|
|
|
|
|
α/β = 9:1 |
|
|
|
|
17 |
||
|
|
|
|
|
|
|
|
|
|
|
|
|||
1) NH3, 0 °C |
|
|
|
|
|
|
HO |
|
O O |
|
|
|
|
|
O |
|
AcO |
|
|
|
|
|
|
|
|
|
|
|
|
P |
O |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
O |
|
|
|
|
|
AcO |
|
|
|
||
Cl |
O |
AcO |
|
|
|
|
19 |
|
O |
|
|
|||
2) N-methylimidazole, |
AcO |
|
O |
|
O |
cat. TMSOTf, -78 °C |
AcO |
O |
O O |
|||||
|
|
|
|
O |
P O |
AcO |
O |
|
|
|||||
r.t., 69% |
|
|
O |
|
|
56% |
|
|
|
|||||
|
|
|
|
OAc O |
|
|
|
|
|
|
|
|||
|
|
AcOOAc |
|
|
|
O |
OAc |
|
||||||
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
AcOOAc |
|
|||||
|
|
|
|
α/β = 20:1 |
|
|
|
|
|
20 |
|
|||
|
|
|
|
18 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 6.3 Syntheses of Shimofuridin analog and fucosidase substrate.
The coupling to hindered acceptor sites as in 32 underscores the power of glycosyl phosphate donors in oligosaccharide synthesis. This efficiency was further illustrated in the extremely high-yielding synthesis of the H type II blood group determinant.33 2-O-benzoyl galactose phosphate 33 was coupled to the hindered C4 position of glucosamine acceptor 32 in 95% yield. Deprotection gave disaccharide acceptor 34, which was α-fucosylated with complete regioand stereoselectivity to furnish protected H type II antigen 37 in 93% yield (Scheme 6.6).
Fucosyl phosphate 36 also very efficiently glycosylated the C4 position of acceptor 32. The orthogonality of the activating conditions of thioglycosides and glycosyl phosphate donors was exploited in the synthesis of trisaccharide glycal 41 (Scheme 6.7A).32 Furthermore, the different reactivity of α- and β-glycosyl phosphates was

|
|
|
|
|
|
|
|
|
6.2 |
|
GLYCOSYL PHOSPHATE DONORS |
121 |
|||||
|
|
|
|
|
|
1) DMDO, 0 °C |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
1 |
|
2) HOP(O)(OR5)2, -78 °C |
|
|
1 |
|
|
|
|
|
|||
|
|
R2O |
OR |
3) R4-Cl, DMAP, 0 °C |
|
OR |
O |
|
|
|
|||||||
|
|
O |
|
|
R2O |
O |
|
|
|
||||||||
|
|
R3O |
|
|
|
|
|
|
R3O |
|
O P OR5 |
|
|
|
|||
|
|
|
|
57-86 % |
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
4 |
5 |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
R |
O |
OR |
|
|
|
|
|
R1, R2, R3 = Bn, Piv, TIPS, TBS |
|
|
|
|
R4 = Piv, TES, Bz |
|
|
|
||||||||
|
|
21 |
|
|
|
|
|
|
|
R5 = Bn, Bu |
22 |
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
1) DMDO, 0 °C |
|
|
|
|
|
1) DMDO, 0 °C |
|
|
|
|
|
|||
|
|
|
2) HOP(O)(OBn)2, |
|
|
|
|
2) HOP(O)(OBn)2, |
|
|
|
||||||
|
OBn |
|
-78 °C, THF |
|
|
|
OBn |
|
-78 °C, toluene |
OBn |
|
O |
|||||
|
|
3) PivCl, DMAP, 0 °C |
|
|
|
3) PivCl, DMAP, 0 °C |
|
||||||||||
BnO |
O |
|
|
BnO |
O |
|
O |
|
|||||||||
|
|
|
|
|
|
|
|
|
|
BnO |
O P OBn |
||||||
BnO |
|
O |
71% |
|
|
|
BnO |
|
84% |
|
|
BnO |
|
||||
|
PivOO P OBn |
|
|
|
|
23 |
|
|
|
PivO |
|
OBn |
|||||
α/β |
|
OBn |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
= 10:1 |
|
|
|
|
|
|
|
|
|
|
|
α/β = 1:17 |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
24 |
|
|
|
|
|
|
|
|
|
|
|
|
|
25 |
|
|
|
Scheme 6.4 One-pot synthesis of glycosyl phosphates from glycals.
illustrated in the synthesis of trisaccharide 44 without any protecting group manipulations (Scheme 6.7B).34
Crich had shown that anomeric α-triflates or close ion pairs are formed on activation of suitably protected glycosyl sulfoxides with excess triflic anhydride, leading to β-selective mannosylation reactions.35 The observed β-selectivity in reactions involving glucosyl phosphates even in the absence of a participating C2 group and observations by Singh36 in reactions of a cyclic glucosyl propane-1,3-diyl phosphate with primary acceptors suggest that these reactions may proceed via α-triflate intermediates. Guided by this model, attempts were directed at the selective creation of β-mannosides employing glycosyl phosphates. The formation of these cis-glycosidic linkages, present in the core region of N-linked glycoproteins,37 still constitutes a major challenge for the synthetic chemist. α-Mannoside formation is
|
|
30, TMSOTf, |
BnO |
|
|
31, TMSOTf, |
|
|
|
|
|
|
|
O |
-78 °C, 94% |
|
BnO |
|
|||
|
|
|
-78 °C, 83% |
O |
|
|
||||
|
BnO |
|
BnO |
O P OBu |
|
|
O |
|||
|
|
|
|
BnO |
||||||
BnO |
O |
|
BnO |
|
|
|
||||
|
|
|
|
|
BnO |
O |
||||
|
BnO |
OMe |
|
|
PivO |
OBu |
|
|
PivO O |
|
BnO |
|
|
|
|
|
|||||
O |
O |
|
|
26 |
|
|
|
|||
BnO |
|
|
BnO |
|
|
|
|
|
O |
|
BnO |
PivO |
|
|
BnO |
O |
|
|
|
|
O |
|
30, TMSOTf, |
BnO |
|
O |
31, TMSOTf, |
|
OO |
|||
|
28 |
PivOO |
|
|||||||
|
|
P OBu |
-20 °C, 87% |
|
29 |
|||||
|
|
|
-20 °C, 73% |
|
|
OBu |
|
|
|
|
|
|
|
|
|
27 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BnO |
|
|
|
O |
OH |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
O |
|
|
||
|
|
BnO |
O |
OMe |
|
|
O |
|
|
|
|
|
BnO |
|
|
OO |
|
||||
|
|
|
HO |
|
|
|
|
|
||
|
|
|
30 |
|
|
|
31 |
|
|
|
Scheme 6.5 Glycosyl phosphates functioning as glycosyl donors in β-selective formation of glycosidic linkages.

122 SOLID-PHASE OLIGOSACCHARIDE SYNTHESIS
|
|
|
|
BnO |
OBn |
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
BnO |
|
O P OBu |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
BzO |
|
OBu |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
33 |
|
|
BnO |
OBn |
|
|
|
|
|
|
|
OBn |
|
TBSOTf, -50 °C- -30 °C |
|
|
OBn |
|
NaOMe, MeOH |
|||||||
|
|
|
|
|
|
||||||||||
HO |
O |
O |
|
|
95% |
BnO |
O |
O |
|
O O |
0 °C, 90% |
||||
BnO |
|
|
|
|
|
|
|
|
|
|
|||||
CbzHN |
|
|
|
|
|
|
|
BzO |
BnO |
CbzHN |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
32 |
|
|
|
|
PivOOPiv |
|
|
34 |
|
|
|
|
|
|
|
|
|
|
|
|
OBn O |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O P OBu |
|
BnO |
OBn |
|
|
|
||
|
|
|
|
|
|
|
36 |
OBu |
|
|
OBn |
|
|||
BnO |
OBn |
|
|
|
|
|
|
|
|
O |
|
|
|||
|
OBn |
|
|
|
TBSOTf, -50 °C- -30 °C |
|
BnO |
|
O |
O |
O |
||||
|
O |
|
|
|
|
|
|
|
|||||||
|
O |
O |
|
|
|
93% |
|
|
|
O |
HO |
|
|||
BnO |
|
O |
|
|
|
|
|
|
|
|
CbzHN |
|
|||
HO |
HO |
|
|
|
|
|
|
|
O |
|
|
|
|||
|
CbzHN |
|
|
|
|
|
|
|
OBn |
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
37 |
|
|||
|
|
35 |
|
|
|
|
|
|
|
PivOOPiv |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
||||
Scheme 6.6 α-Fucosylation of a disaccharide acceptor with complete regio/stereoselctivity.
A
B
HO
BnO
42
|
|
|
|
|
BnO |
|
|
HO |
OPiv |
26, TMSOTf, |
BnO |
O |
O |
||
|
BnO |
||||||
BnO |
O |
-78 °C, 83% |
|
|
|||
|
|
PivO |
OPiv |
||||
BnO |
SEt |
|
|
|
|
||
|
|
|
|
|
BnO |
O |
|
|
38 |
|
|
|
|
||
|
|
|
|
|
BnO |
SEt |
|
|
|
|
|
|
|
|
|
|
OBn |
|
|
|
|
|
39 |
HO |
O |
BnO |
O |
|
|
|
|
BnO |
40 |
BnO |
|
O |
|
|
|
|
BnO |
|
|
|
|
||
|
PivO |
|
|
OPiv |
|
||
MeOTf, DTBP, |
|
|
|
|
|||
0 °C, 68% |
|
BnO |
|
|
O |
OBn |
|
|
|
|
BnO |
|
|
||
|
|
|
|
|
|
O |
O |
|
|
|
41 |
|
BnO |
|
|
|
|
|
|
|
|
||
OBn |
|
|
|
|
OBn |
|
OBn |
O |
|
|
BnO |
O |
|
||
|
26, TMSOTf, -78 °C |
O |
O |
||||
|
O |
|
BnO |
|
|
||
PivO |
|
PivO BnO PivO O |
|||||
|
|
|
|
||||
O P OBu |
|
|
|
|
|
O P OBu |
|
|
OBu |
|
|
|
43 |
||
|
|
|
|
OBu |
|||
|
|
|
|
|
|
|
|
|
|
|
OBn |
|
OBn |
|
|
|
|
BnO |
O |
|
|
|
|
|
|
O |
O |
|
|
||
|
|
|
O |
|
|||
|
|
BnO |
PivO |
BnO |
PivO O |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
31, TMSOTf, -20 °C |
|
|
O |
|
O |
|
|
|
|
|
|
|||
|
|
|
|
|
|
O |
|
|
|
|
~40% overall |
|
|
||
|
|
|
|
|
O |
||
|
|
|
|
44 |
|
|
|
Scheme 6.7 Orthogonal glycosylations to fashion trisaccharides.
6.2 GLYCOSYL PHOSPHATE DONORS 123
|
|
|
|
|
cat. TMSOTf, ROH |
BnO |
OBn |
||
|
|
|
|
|
-78-0 °C, CH2Cl2 |
||||
|
|
|
|
|
BnO |
O |
|||
|
|
|
|
|
|
|
BnO |
|
|
BnO |
OBn |
|
50-96% |
|
OR |
||||
|
|
46 |
|||||||
BnO |
O |
O |
|||||||
|
|||||||||
BnO |
|
|
|
||||||
|
O P |
O |
|
|
|||||
|
|
|
|
||||||
|
|
O |
|
1.5 eq TMSOTf, ROH, |
BnO |
OBn |
|||
|
45 |
|
|
-78-0 °C, CH2Cl2 |
|||||
|
|
|
|
BnO |
O |
||||
|
|
|
|
|
|
|
|||
|
|
|
46-78% |
|
BnO |
OR |
|||
|
|
|
|
|
|||||
|
|
|
|
|
|
|
α/β = ~1:1 |
||
|
|
|
|
|
|
|
47 |
|
|
Scheme 6.8 Formation of α-mannosides on cyclic mannosyl phosphate activation.
favored principally for steric reasons and the anomeric effect. As expected, only α-mannosides were formed on activation of cyclic mannosyl phosphate 45 with catalytic amounts of TMSOTf in the presence of various glycosyl acceptors (Scheme 6.8).38 When excess activator was employed, Singh observed considerable increase of β-mannoside formation.
Better β-selectivities were achieved using mannosyl diphenyl phosphate 48 and hindered acceptor 49 under activation with excess TMSOTf to form preferentially the β-mannoside (β:α = 3:1) (Scheme 6.9).39 The selectivity of the reaction was found to be strongly substrate-dependent. The influence of the acceptor conformation and reactivity on the selectivity of β-mannosylation reactions had also previously been reported by Crich.40 When the couplings were carried out in a coordinating solvent such as acetonitrile, the selectivity of the mannoside formation was completely reversed (α:β = 5.5:1) (Scheme 6.9). Interestingly, no solvent dependence was found in the coupling of 2-deoxy-2-azido glucosyl phosphate donor 51 with hindered acceptor 49, but rather high β-selectivity and yields were observed in both solvents.39
|
|
|
OBn |
|
|
|
|
|
BnO |
|
OBn |
BnO |
|
O |
|
|
|
|
BnO |
|
OMe |
|
OBn |
|||
BnO |
|
O |
49 |
OH |
|
|||
|
BnO |
O |
||||||
BnO |
|
O |
|
|||||
|
TMSOTf, -78 °C |
OMe |
||||||
|
|
O P OPh |
|
BnO |
||||
48 |
|
|
|
|
BnO |
OBn |
O |
|
|
OPh |
CH2Cl2 |
: 88%; α/β = 1:3 |
BnO |
O |
|
||
|
|
|
||||||
|
|
|
|
|||||
|
|
|
MeCN |
: 83%; α/β = 5.5:1 |
BnO |
|
|
|
|
|
|
|
50 |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OBn |
BnO |
|
O |
49, TMSOTf, -40 °C |
|
BnO |
O |
||
|
|
OMe |
||||||
BnO |
O |
O P OBu |
BnO |
BnO |
||||
|
|
|
O |
O |
||||
BnO |
|
|
|
|
BnO |
|||
N3 OBu |
|
: 77%; α/β = 1:4 |
|
|||||
51 |
CH2Cl2 |
BnO |
N3 |
|
||||
|
|
MeCN |
: 61%; α/β = 1:4 |
|
52 |
|||
Scheme 6.9 Formation of β-mannosides using glycosyl phosphates.
124 SOLID-PHASE OLIGOSACCHARIDE SYNTHESIS
6.2.2 Special Applications of Glycosyl Phosphates in Oligosaccharide
Synthesis
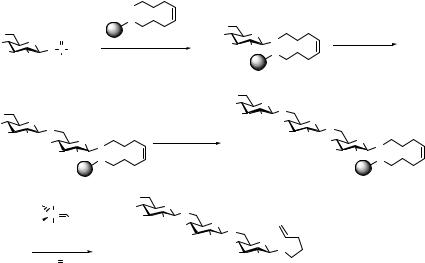
As outlined above, glycal derived glycosyl phosphates have proved to be very easily accessible and effective glycosyl donors. They require only liquid, non-toxic activators, and generally no addition of molecular sieves is necessary. Therefore, glycosyl phosphates lend themselves particularly well to the synthesis on solid support. We also reported on the high-yielding solid-phase syntheses of oligosaccharides employing a novel octenediol linker (Scheme 6.10).41 β-(1→6)-Linked triglucoside 58 was obtained in 35% overall yield over seven steps using 3 equiv of glycosyl phosphate donor 53 for each coupling step. The glycosylations were carried out at –50°C for one hour followed by removal of the 6-O-triisopropylsilyl (TIPS) protecting group with tetrabutylammonium fluoride (TBAF). The trisaccharide was finally released from the support by cross-metathesis using Grubbs’ catalyst under an atmosphere of ethylene to yield n-pentenyl glycoside
58.
Using a similar reaction sequence β-(1→4)-glucosidic linkages were very effectively installed on the support using 4-O-TBS-protected glucosyl phosphate donor 59. Thus, repetitive glycosylation and deprotection furnished trisaccharide 62 in 53% overall yield from 54 after cleavage from the resin (Scheme 6.11).
C-Glycosidic linkages are present in various natural products and may inhibit glycosidases due to the structural analogy to their O-counterparts.42 Glycosyl phosphates 63 and 48 served successfully in the α-selective synthesis of aryl
|
|
|
|
|
HO |
|
|
|
|
|
1) TBAF |
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
||
|
|
|
|
|
|
TIPSO |
|
|
|
2) 53, TMSOTf, |
|||
TIPSO |
|
|
|
|
|
|
|
O |
|
||||
O |
O |
|
54 |
|
BnO |
|
O |
|
-50 °C |
|
|||
BnO |
O P OBu |
|
|
|
BnO |
PivO |
|
|
|
||||
|
|
|
|
|
|
|
|
||||||
BnO |
PivO |
OBu |
|
TMSOTf, -50 °C |
55 |
|
|
O |
|
|
|
||
|
53 |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TIPSO |
O |
|
|
|
|
|
|
|
|
|
|
|
|
BnO |
|
O |
|
|
|
|
TIPSO |
|
|
|
|
|
1) TBAF |
BnO |
|
|
|
|
||
O |
|
|
|
|
PivOBnO |
O |
|
|
|||||
BnO |
O |
|
|
|
2) 53, TMSOTf, |
|
|
|
|||||
BnO |
|
|
|
|
|
|
BnO |
O |
|
|
|||
PivO |
|
O |
|
|
-50 °C |
|
|
|
PivO |
O |
|
||
|
BnO |
O |
|
|
|
|
|
|
BnO |
O |
|||
|
|
|
|
|
|
|
|
|
|||||
|
BnO |
PivO |
|
|
|
|
|
|
BnO |
PivO |
|||
|
O |
|
|
|
|
|
57 |
O |
|||||
|
56 |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cl |
P(Cy)3 |
|
TIPSO |
O |
|
|
|
|
|
|
|
|
|
|
BnO |
|
|
|
|
|
|
|
||||
|
|
Ru |
|
|
O |
|
|
|
|
|
|
|
|
|
|
Ph |
|
BnO |
|
|
|
|
|
|
|
||
|
Cl |
|
PivO |
O |
|
|
|
|
|
|
|||
|
|
P(Cy)3 |
|
|
BnO |
O |
|
|
|
|
|
|
|
|
|
|
|
BnO |
|
|
|
|
|
|
|||
|
(Grubbs' cat.) |
|
|
PivO BnO |
|
O |
O |
|
|
|
|||
|
|
|
|
|
|
58 |
BnO |
PivO |
|
|
|
||
|
H2C CH2 |
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|||
34% overall
Scheme 6.10 Solid-phase synthesis of oligosacchraides using an octenediol linker.

6.2 GLYCOSYL PHOSPHATE DONORS 125
|
BnO |
O |
O |
|
|
|
|
|
TBSO |
|
|
|
|||
|
O P OBu |
|
|
|
|||
|
BnO |
PivO |
|
|
|
||
|
OBu |
BnO |
|
|
|||
HO |
59 |
|
|
||||
|
O |
|
|||||
|
|
TBSO |
O |
||||
|
|
|
|
||||
O |
TMSOTf, -50 °C |
BnO |
PivO |
||||
O |
|||||||
|
|
|
|
|
|||
54 |
|
|
|
|
|
||
|
|
|
60 |
|
|
||
1)TBAF
2)59, TMSOTf,
-50 °C |
|
BnO |
O BnO |
|
BnO |
|
|
||
|
TBSO |
O |
|
|
|||||
reiterate |
|
|
|
O |
O |
|
|||
|
|
|
BnO |
PivO |
|
O |
O |
||
|
|
|
|
BnO |
PivO |
BnO |
PivO |
||
|
|
|
|
|
|
O |
|||
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
61 |
|
|
|
Cl |
P(Cy)3 |
|
|
|
|
|
|
|
|
Cl |
Ru |
Ph |
|
|
|
|
|
|
|
|
BnO |
|
|
|
|
|
|
||
|
P(Cy)3 |
O |
BnO |
|
BnO |
|
|
||
(Grubbs' cat.) |
TBSO |
O |
O |
|
|||||
|
|
|
BnO |
|
O |
|
O |
|
|
|
|
|
PivO |
BnO |
|
|
O |
||
H2C CH2 |
|
PivO |
BnO |
PivO |
|||||
|
|
|
|
||||||
|
|
|
|
|
|
||||
53 % overall
62
Scheme 6.11 Solid-phase synthesis of a β(1→4) linked triglucoside.
O-glycosides at 0°C, which, on warming to room temperature, rearranged to C-aryl glycosides (Scheme 6.12). The preference for the equatorial position of bulky aromatic C-aglycons was evident and had been observed before.42 While couplings employing 2-O-acyl protected glycosyl phosphates were unsatisfactory in most cases,
|
OBn |
|
BnO |
O |
|
BnO |
BnO |
O |
|
|
O P OPh
63 OPh
BnO OBn
BnO 
 O
O
BnO  O
O
O P OPh
48
OPh
OH
64
TMSOTf, 0 °C- r.t.
64, TMSOTf, 0 °C- r.t.
|
OBnHO |
|
OBn |
|
|
BnO |
O |
||
BnO |
O |
BnO |
BnO |
|
BnO |
BnO |
+ |
||
O 66 |
||||
|
|
|||
|
65 |
|
|
|
|
60% |
|
9% |
BnO OBn
BnO 
 O
O
BnO
OH
75% 67
Scheme 6.12 Rearrangement to C-aryl glycosides from aryl O-glycosides following α-selective synthesis using glycosyl phosphates.

126 SOLID-PHASE OLIGOSACCHARIDE SYNTHESIS
PivO |
O |
|
O |
1M LiClO4, ROH |
PivO |
|
|||
PivO |
|
|
|||||||
O |
P OBn |
41-68% |
|
|
PivO |
O |
|||
PivO |
|
|
|
||||||
|
|
|
|
|
|||||
|
PivO |
|
OBn |
|
|
|
|
PivO |
OR |
|
|
|
|
|
|
PivO |
|||
|
68 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
69 |
|
|
|
|
|
|
|
|
|
|
|
|
BnO |
O |
|
|
|
1M LiClO4, LiI, |
BnO |
|
||
BnO |
O |
|
|
O |
|||||
BnO |
|
|
|
ROH, 11-63% |
BnO |
||||
|
BnOO P OPh |
|
|
|
|
BnO |
BnOOR |
||
|
63 |
OPh |
|
|
|
|
|
||
|
|
|
|
|
|
|
α/β = 3:1-23:1 |
||
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
70 |
|
Scheme 6.13 Glycoslation of carbohydrate acceptors with glycosyl α-diphenyl phosphate.
perbenzylated phosphate donors gave high yields also with nonaromatic C-glycosyl acceptors.43
The phosphate leaving group also served in samarium(II)iodide-mediated C-glycosidations, as described by Wong.44 A glycosyl anion equivalent, generated via a two-step sequence, added in high yield to a series of carbonyl electrophiles, thereby stereoselectively creating C-glycosidic linkages.
Direct nucleophilic displacements of the phosphate leaving group in inert organic solvents by nucleophiles of the A–B?
C–H type, e. g. phenols, have been described by Schmidt.28 Concentrated solutions of lithium perchlorate in organic solvents enabled similar glycosylations under neutral conditions employing various acceptors, including carbohydrates. While 2-O-acylated glycosyl β-phosphates gave exclusively β-glycosides,45,46 glycosylation reactions employing perbenzylated glycosyl phosphates resulted in varying stereoselectivities depending on the nature of the solvent and the anomeric configuration of the glycosyl donor.45,47 However, glycosylations of carbohydrate acceptors were unselective. This problem was solved by the use of lithium iodide as an additive for the in situ48 generation of glycosyl iodide donors.49 A series of primary and secondary carbohydrate acceptors were glycosylated with glycosyl α-diphenyl phosphate 63 in the presence of lithium iodide resulting in α/β-selectivities of up to 23:1 (Scheme 6.13).
In a similar way glycosyl diphenyl phosphates have been used in the stereoselective preparation of glycosyl azides on SN2-type displacement of the phosphate group by sodium azide.27
6.3 OTHER PHOSPHOROUS(V) GLYCOSYL DONORS
A variety of phosphate analogs have been synthesized and evaluated as glycosyl donors. Glycosyl phosphoramidimidates 73 are readily available from glycosyl phosphoramidites via a Staudinger reaction with phenyl azide (Scheme 6.14). Bearing

|
|
6.3 |
OTHER PHOSPHOROUS(V) GLYCOSYL DONORS 127 |
|||||
RO |
OR |
EtOP(NiPr2)2 |
, r.t. RO |
OR |
|
|
PhN , 45 °C |
|
O |
O |
|
|
|||||
RO |
|
|
RO |
|
OEt |
3 |
||
|
OH |
|
|
O P |
|
|||
|
|
|
|
|
||||
|
RO |
|
|
RO |
NiPr2 |
|
||
|
|
|
|
|
|
|||
|
71 |
|
|
|
|
72 |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
R'OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OR |
|
1 eq TMSOTf |
|
OR |
|
|
|
RO |
|
or BF3·Et2O |
RO |
|
|
|||
O |
NPh |
|
|
|||||
O |
|
R = Me, Bn |
||||||
RO |
|
-78 °C |
|
|||||
|
O P OEt |
|
RO |
O'R |
||||
RO |
|
|
RO |
|
||||
|
NiPr2 |
|
|
|
|
|
||
|
|
|
|
|
|
|
||
|
73 |
|
|
|
74 |
|
|
|
|
|
|
|
|
|
|
||
Scheme 6.14 Synthesis of glycosyl phosphoramidimidates from glycosyl phosphoramidites.
a nonparticipating group at C2, glycosyl phosphoramidimidates have been shown to undergo glucosylation and galactosylation reactions with primary acceptor glycosides exhibiting α:β-selectivities of ≤1:20 in some cases, although yields and selectivities generally leave room for improvement.50 The synthesis of these glycosyl donors proved advantageous since no workup or purification was required for the crude products from the Staudinger reaction.
Glycosyl phosphorimidate donors were obtained by the synthetic sequence outlined in Scheme 6.14. These glycosyl donors were coupled with primary acceptor glycosides in moderate to excellent yields, and depending on the reaction conditions (Scheme 6.15) either α- or β-selectivities were observed.51 β-Selective mannosylations and glycosylation on reaction of secondary hydroxyl containing acceptors did not prove feasible.
The synthesis and application of glycosyl dimethylthiophosphate donors has also been reported.52 These donors were prepared from the reducing sugars in moderate to good yield and exhibit considerable shelf stability. Glycosylation reactions with a variety of primary and secondary glycosyl acceptors suffered from poor stereoselectivity and required silver triflate as promoter to achieve reasonable coupling yields (Scheme 6.16).
Hashimoto and Ikegami have introduced glycosyl phosphoroamidates as glycosyl donors that combine the advantages of long shelf life with great efficiency in
|
|
|
|
R'OH, 4 Å MS, 40 °C, |
|
OR |
|
||
|
|
|
lutidinium p-toluenesulfonate/TBAI |
RO |
|
||||
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
RO |
α/β = 4:1-10:1 |
|
RO |
OR |
|
|
|
|
|
RO OR' |
||
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|||
O |
NPh |
76 |
|
||||||
RO |
O |
P OEt |
|
|
|||||
|
|
|
|||||||
|
|
|
|
||||||
RO |
OEt |
|
|
R = Me, Bn |
|||||
|
|
|
|
||||||
75 |
|
|
R'OH, 1.2 eq TMSOTf |
|
OR |
|
|||
|
|
EtCN, -78 °C |
RO |
|
|||||
|
|
|
|
O |
α/β = 1:5-1:35 |
||||
|
|
|
|
|
|
|
RO |
||
|
|
|
|
|
|
|
RO OR' |
|
|
|
|
|
|
|
|
|
77 |
|
|
|
|
|
|
|
|
|
|
|
|
Scheme |
6.15 |
Coupling of glycosyl phosphorimidate |
donors with |
primary acceptor |
|||||
glycosides.
