
Multiple Bonds Between Metal Atoms / 04-Molybdenum Compounds
.pdf
Molybdenum Compounds 79
Cotton
The 1:2 adduct Mo2(O2CCF3)4(bpy)2 is an unusual complex since it has a centrosymmetric structure consisting of unbridged neutral Mo2 units with four δ1-O2CCF3 groups and chelating bpy ligands.55 It is prepared by reacting acetone solutions of Mo2(O2CCF3)4 and bpy in a 1:2 ratio and from the thermal and photochemical conversion of [Mo2(µ-O2CCF3)2(bpy)2](O2CCF3)2. The correct Mo–Mo distance, however, is 2.129(1) Å, not 2.077(1) Å.
Another important group of adducts are those involving monoanionic ligands, for example Mo2(O2CH)4·KCl.35 It is formed from solutions of K4Mo2Cl8·2H2O in 90% formic acid and has been shown to contain intact Mo2(O2CH)4 units which are linked by weak chloride bridges to give an infinite zig-zag chain structure. However, a more extensive series of halide-containing complexes have been obtained by the use of organic cations. One of the earliest studies was that described by Garner and Senior,110 who isolated 1:1 adducts of the type Et4N[Mo2(O2CCF3)4X], where X = Cl, Br, I, CF3CO2 and SnCl3−, together with certain 1:2 adducts (Et4N)2[Mo2(O2CCF3)4X2], where X = Br and I, by mixing dichloromethane solutions of Mo2(O2CCF3)4 and the appropriate Et4NX salt. These adducts appear to be more stable than those formed by neutral donors, an observation that was attributed to the lattice energy of the salts. A series of structural studies on the complexes (Bu4N)2[Mo2(O2CCF3)4X2] (X = Cl or Br)70,71 have confirmed that they all have structures with short Mo–Mo distances and long axial Mo–X bonds. For example, in the case of (Ph4P)2[Mo2(O2CC6H5)4Cl2]70 the Mo–Cl distance of 2.88 Å is similar to that in Mo2(O2CH)4·KCl (c. 2.86 Å).35 The aforementioned complexes are prepared directly from the parent Mo2(O2CR)4 compounds. The azido complex (Ph4P)2[Mo2(O2CC6H5)4(N3)2] has also been prepared, but upon its dissolution in dichloromethane N2 is evolved and the dichloride adduct is formed.70
A useful spectroscopic probe of the existence of significant Mo–Lax interactions is provided by the reduction in the Raman active ι(Mo–Mo) mode upon complex formation. For example, ι(Mo–Mo) of solid Mo2(O2CCH3)4 and Mo2(O2CCF3)4 occur at 406 and 397 cm−1, respectively,12,92 and both frequencies shift approximately −30 cm−1 upon formation of the bis-pyridine adducts.29 This Raman frequency shift, together with variations in the electronic absorption spectra, has been used to probe the nature of the interaction of neutral or anionic base ligands with a variety of dimolybdenum(II) carboxylates.29,36,49,100,101,107,110
4.1.3 Other compounds with bridging carboxyl groups
There are many compounds in which one, two, or three, but not four carboxyl groups span the Mo24+ core. Those with known structures are listed in Table 4.2. Compounds of this class are generally obtained by partial replacement of RCO2 ligands in Mo2(O2CR)4 compounds. The overwhelming majority contain acetate ion, but compounds containing PhCO2−, CF3CO2−, Me3CCO2− and others are also known.
There are very few examples of dimolybdenum(II) complexes in which three carboxylate groups are present. Attempts to prepare (Et3N)2[Mo2(O2CCF3)4Cl2] led to the unplanned discovery110 of (Et3N)2[Mo2(O2CCF3)3Cl3]. Other complexes with three carboxylate ligands are the various salts of composition MI2[Mo2(O2CH)3Cl3]·HCl·2H2O (MI = NH4, K, Rb or Cs) and Cs[Mo2(O2CH)3(SO4)]·2H2O, which have been isolated by reacting mixtures of NH4O2CH and MICl with K4Mo2Cl8, (NH4)5Mo2Cl9·H2O or (NH4)4[Mo2(SO4)4]·2H2O.111,112 Characterization of these complexes is based primarily on their vibrational spectra111,112 but in the case of MI2[Mo2(O2CH)3Cl2]·Cl·2H2O (M = NH4 or Rb) full crystal structure data are available.112,113 The eclipsed [Mo2(O2CH)3Cl2]− anions have Mo–Mo distances of 2.099(3) Å and 2.106(3) Å, respectively, but the nature of the axial ligands (Cl− and/or H2O) could not be discerned because of a disorder problem.
Table 4.2. Other Compounds with Carboxylate Ligands
|
|
Compound a |
Crystal |
Virtual |
r(Mo–Mo) (Å) |
Twist |
ref |
|
|
sym. |
sym.b |
Angle (°)c |
|||
|
|
|
|
|
|||
[Mo2(O2CCH3)3(S2CPEt3)(OPEt3)]BF4 |
1 |
Cs |
2.138(1) |
0 |
114 |
||
Mo2(O2CCH3)3(BAII)·C6H6 |
|
|
1 |
Cs |
2.106(1) |
0 |
115 |
(Ph4As)2[Mo2(O2CCH3)2Cl4]·2CH3OH |
1 |
D2h |
2.086(2) |
50 |
116,117 |
||
cis-Mo2(O2CCH3)2(NCCH3)4(SO3CF3)2·2CF3SO3H·THF |
m |
C2v |
2.132(4) |
0 |
118 |
||
cis-[Mo2(O2CCH3)2(NCCH3)6](BF4)2 |
m |
C2v |
2.134(2) |
0 |
118 |
||
trans-[Mo2(O2CCH3)2(µ-dmpe)2](BF4)2·CH3CN |
¯ |
D2h |
2.099(1) |
0 |
119 |
||
1 |
|||||||
|
|
|
¯ |
D2h |
2.096(1) |
0 |
|
|
|
|
1 |
|
|||
trans-Mo2(O2CCH3)2(CH2SiMe3)2(PMe3)2 |
¯ |
C2h |
2.098(1) |
0 |
120 |
||
1 |
|||||||
trans-Mo2(O2CCH3)2(CH2Ph-p-Me)2(PMe3)2 |
¯ |
C2h |
2.108(2) |
0 |
121 |
||
1 |
|||||||
|
|
|
¯ |
C2h |
2.107(1) |
0 |
|
|
|
|
1 |
|
|||
trans-Mo2(O2CCH3)2(OSiMe3)2(PMe3)2 |
¯ |
C2h |
2.114(1) |
0 |
122 |
||
1 |
|||||||
trans-Mo2(O2CCH3)2Cl2(PBu |
n |
¯ |
C2h |
2.099(1) |
0 |
123 |
|
|
3)2 |
1 |
|||||
trans-Mo2(O2CCH3)2Cl2(PPh3)2 |
¯ |
C2h |
2.091(1) |
0 |
124 |
||
1 |
|||||||
trans-Mo2(O2CCMe3)2Cl2(PEt3)2 |
¯ |
C2h |
2.098(1) |
0 |
125 |
||
1 |
|||||||
cis-Mo2(O2CCMe3)2Cl2(PEt3)2 |
|
1 |
C2 |
2.113(1) |
0 |
125 |
|
trans-Mo2(O2CCH3)2Cl2(Ph2Ppy)2·2CH2Cl2 |
¯ |
C2h |
2.190(1) |
0 |
126 |
||
1 |
|||||||
Mo2(O2CCH3)Cl3(PMe3)3·0.5C7H8 |
m |
Cs |
2.121(2) |
0 |
127 |
||
cis-Mo2(O2CCH3)2(acac)2 |
|
|
1 |
C2v |
2.129(1) |
0 |
116 |
cis-Mo2(O2CCH3)2[PhNC(CH3)CHC-(CH3)O]2 |
1 |
C2 |
2.131(1) |
0 |
128 |
||
cis-Mo2(O2CCH3)2[(pz)2BEt2]2 |
1 |
Cs |
2.129(1) |
2.6 |
129 |
||
cis-Mo2(O2CCH3)2[(pz)3BH]2 |
|
1 |
Cs |
2.147(3) |
3.4 |
129 |
|
cis-Mo2(O2CCH3)2(pdc)2(OPPh3)·1.5C6H6 |
1 |
Cs |
2.134(1) |
0 |
114 |
||
trans-Mo2(O2CCH3)2{[(2,6-xylyl)N]2CCH3}2(THF)2·2THF |
¯ |
D2h |
2.107(1) |
0 |
130 |
||
1 |
|||||||
trans-Mo2(O2CCH3)2[o-(Me2N)C6H4CH2]2 |
¯ |
Ci |
2.065(1) |
0 |
131 |
||
1 |
|||||||
trans-Mo2(O2CCH3)2(7-azaindolyl)2·2DMF |
¯ |
C2h,C2v |
2.112(1) |
0 |
132 |
||
1 |
|||||||
|
i |
)4]2 |
¯ |
C2h |
2.079(1) |
0 |
133 |
trans-Mo2(O2CCH3)2[Al(OPr |
1 |
||||||
|
80 |
|
4 Chapter |
|
Bonds Multiple |
|
||
|
|
Atoms Metal Between |
Compound a |
Crystal |
Virtual |
r(Mo–Mo) (Å) |
Twist |
ref |
|
sym. |
sym.b |
Angle (°)c |
||||
|
|
|
||||
cis-[Mo2(O2CCH3)2(en)2](ax-en)(O2CCH3)2·en |
m |
C2v |
2.125(1) |
0 |
134(a) |
|
Mo2(O2CCH3)(ambt)3·2THF |
1 |
Cs |
2.093(3) |
1.9 |
135 |
|
Mo2(O2CCH3)[(PhN)2CCH3]3 |
1 |
C2v |
2.082(1) |
0 |
130 |
|
(C3N2H5){Mo2(O2CCH3)[CH3Ga(C3N2H3)O]4}·2THF |
mm |
C2v |
2.127(1) |
0 |
136 |
|
trans-[Mo2(µ-O2CCH3)2(µ-dppma)2(CH3CN)2](BF4)2·4CH3CN |
¯ |
D2h |
2.113(1) |
Zero |
137 |
|
1 |
||||||
|
|
|
2.130(1) |
|
|
|
|
|
|
|
|
|
|
trans-[Mo2(µ-O2CCH3)2(µ-dppma)2(BF4)2]·2CH2Cl2 |
¯ |
D2h |
2.115(1) |
Zero |
137 |
|
1 |
||||||
|
|
|
2.111(1) |
|
|
|
|
|
|
|
|
|
|
trans-[Mo2(µ-O2CCH3)2(µ-dppma)2(NCC(CH3)3)2](BF4)2·0.5(C4H10O,C6H14) |
1 |
D2h |
2.115(1) |
0.8 |
137 |
|
|
|
|
2.116(1) |
7.8 |
|
|
|
|
|
|
|
|
|
trans-[Mo2(µ-O2CCH3)2(µ-dppma)2(NCC6H5)2](BF4)2 |
¯ |
D2h |
2.131(1) |
Zero |
138 |
|
1 |
||||||
trans-[Mo2(µ-O2CCH3)2(µ-dppma)2(NCC6H4C>CH)2]((BF4)2 |
¯ |
D2h |
2.131(1) |
Zero |
137 |
|
1 |
||||||
Mo2(O2CCH3)(triphos)Br3·2CH2Br2 |
1 |
C1 |
2.132(3) |
13.6 |
139 |
|
trans-[Mo2(O2CCH3)2((Ph2PCH2)2PPh)2](BF4)2·2CH2Cl2 |
¯ |
C2h |
2.119(3) |
Zero |
140 |
|
1 |
||||||
trans-[Mo2(O2CCH3)2(dpmp-O)2](BF4)2·2CH2Cl2 |
¯ |
C2h |
2.141(2) |
Zero |
140 |
|
1 |
||||||
dpmp-O = Ph2PCH2PPhCH2P(O)Ph2 |
|
|
|
|
|
|
cis-[Mo2(O2CCH3)2(dpnapy-N,P)2](BF4)2·C7H8·2CH2Cl2 |
1 |
C2 |
2.119(1) |
NR |
141 |
|
trans-[Mo2(O2CCH3)2(dpnapy-N,P)2](BF4)2·5C6H6 |
¯ |
C2h |
2.099(2) |
Zero |
141 |
|
1 |
||||||
trans-[Mo2(O2CCH3)2(dppma)2(NC5H4CMe3)2](BF4)2·CH2Cl2 |
¯ |
D2h |
2.150(1) |
Zero |
142 |
|
1 |
||||||
Mo2Cl2(O2CCH3)2(py)2·CH2Cl2 |
1 |
C2 |
2.131(1) |
NR |
143 |
|
trans-Mo2(O2CCH3)2[PhC(NSiMe3)2]2 |
2 |
D2h |
2.069(1) |
NR |
144 |
|
cis-Mo2(O2CCH3)2[PhC(NSiMe3)2]2 |
2 |
C2v |
2.124(1) |
NR |
144 |
|
trans-Mo2Cl2(OCCH3)2(dppa)2·2CH3OH |
¯ |
D2h |
2.152(2) |
Zero |
145 |
|
1 |
||||||
trans-[Mo2(O2CCH3)2(dppa)2(CH3CN)2](BF4)2·CH3CN |
¯ |
D2h |
2.133(1) |
Zero |
146 |
|
1 |
||||||
|
|
|
2.136(1) |
|
|
|
|
|
|
|
|
|
|
trans-[Mo2(O2CCH3)2Cl2(dppma)2]·2CH3CN |
2 |
D2h |
2.172(1) |
10 |
147 |
|
trans-[Mo2(O2CCH3)2(µ-dppa)2](BF4)2 |
¯ |
D2h |
2.112(1) |
Zero |
148 |
|
1 |
||||||
[Mo2(O2CCH3)2(pynp)2](BF4)2·3CH3CN |
1 |
C2 |
2.124(1) |
NR |
149 |
Cotton |
|
Compounds Molybdenum |
|
81 |
|

|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Compound a |
Crystal |
Virtual |
r(Mo–Mo) (Å) |
Twist |
ref |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
sym. |
sym.b |
Angle (°)c |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
cis-[Mo2(mphamnp)2(O2CCH3)2]·C5H12 |
|
|
2 |
C2 |
2.097(2) |
NR |
150 |
||||||||||||||||||
Hmphamnp = 2-acetamido-5-methyl-7-phenyl-1,8-naphthyridine |
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
||||||||||||||||||||
[Mo2PtBr2(pyphos)2(O2CCH3)2]2·4CH2Cl2 |
1 |
Cs |
2.096(1) |
4.8[4] |
151 |
||||||||||||||||||||
Mo2(O2CCH3)2(SSiMe3)2(PEt3)2 |
|
|
|
|
¯ |
C2h |
2.110(1) |
Zero |
152 |
||||||||||||||||
|
|
|
|
1 |
|||||||||||||||||||||
Mo2(O2CCH3)2(H2-calix[4]arene)]·THF·C6H6 |
1 |
Cs |
2.126(1) |
~0 |
153 |
||||||||||||||||||||
Mo |
2 |
(O |
2 |
CCH |
3 |
) (Do-OMePhF) |
|
|
|
|
|
1 |
C |
2v |
2.093(1) |
NR |
154 |
||||||||
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
trans-Mo |
2 |
(O |
|
CCH |
) (Do-OMePhF) |
·2CH |
2 |
Cl |
2 |
1 |
D |
2h |
2.108(1) |
NR |
154 |
||||||||||
|
|
|
|
|
|
2 |
|
|
3 |
2 |
|
2 |
|
|
|
|
|
|
|
|
|||||
Mo2(O2CCH3)(Do-OMePhF)Cl2(PMe3)2 |
|
|
|
1 |
C1 |
2.124(1) |
NR |
154 |
|||||||||||||||||
[Bun |
N] |
[Mo |
|
(O CCH |
)(CN) |
] |
|
|
|
|
1 |
C |
2v |
2.114(2) |
3.5 |
155 |
|||||||||
|
|
4 |
|
|
3 |
|
|
|
2 |
2 |
3 |
6 |
|
|
|
|
|
|
|
|
|
|
|||
[Mo2PdCl2(pyphos)2(O2CCH3)2]2·2CH2Cl2·Et2O |
1 |
Cs |
2.083(6) |
NR |
156 |
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.099(6) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
trans-[Mo2(µ-O2CCH3)2(µ -2-(diphenylphosphino)-6-(pyrazol-1-yl)pyridine)2](BF4)2 |
¯ |
C2h |
2.153(1) |
Zero |
157 |
||||||||||||||||||||
1 |
|||||||||||||||||||||||||
[Mo2(O2CCH3)2(dppm)2](BF4)2·3CH3CN |
|
1 |
D2h |
2.132(1) |
NR |
158 |
|||||||||||||||||||
[Mo2(O2CCH3)2(dppe)2](BF4)2 |
|
|
|
|
|
¯ |
C2h |
2.093(1) |
Zero |
158 |
|||||||||||||||
|
|
|
|
|
1 |
||||||||||||||||||||
Mo2(O2CCH3)2(dppee)2](BF4)2·2CH3CN |
|
¯ |
C2h |
2.144(1) |
Zero |
158 |
|||||||||||||||||||
|
1 |
||||||||||||||||||||||||
Mo2Cl3(O2CCH3)(δ3-tetraphos-2)·THF |
|
|
1 |
C1 |
2.126(3) |
13.2 |
159 |
||||||||||||||||||
Mo2(O2CCH3)Cl3(δ3-triphos)·2CH2Cl2 |
|
|
1 |
C1 |
2.121(3) |
11.4 |
159 |
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.134(3) |
11.7 |
|
Mo2(O2CCF3)3Cl(NCC2H5) |
|
|
|
|
|
1 |
Cs |
2.127(2) |
0 |
160 |
|||||||||||||||
Mo2(O2CCF3)2Cl2(NCC2H5)2 |
|
|
|
|
|
1 |
C2 |
2.134(2) |
0 |
160 |
|||||||||||||||
(Bu4N)2[Mo2(O2CCF3)2Br4] |
|
|
|
|
|
1 |
D2h |
2.098(1) |
0 |
58 |
|||||||||||||||
cis-[Mo2(O2CCF3)2(bpy)2](O2CCF3)2 |
|
|
|
1 |
C2v |
2.181(2) |
0 |
55 |
|||||||||||||||||
{[trans-Mo2(O2CCF3)2(µ-dppa)]3(µ6-CO3)(µ2-Cl)3}F·4CH2Cl2·2Et2O |
2 |
D3h |
2.153(1) |
NR |
161 |
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.155(1) |
|
|
{[trans-Mo2(O2CCF3)2(µ-dppa)]3(µ6-CO3)(µ2-Br)3}F·4CH2Cl2·2Et2O |
2 |
D3h |
2.152(1) |
NR |
161 |
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.148(1) |
|
|
|
|
|
|
|
|
||||||||||||||||||||
{[trans-Mo2(O2CCF3)2(µ-dppa)]3(µ6-CO3)(µ2-I)3}F·4CH2Cl2·2Et2O |
2 |
D3h |
2.150(1) |
NR |
161 |
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.154(1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
82 |
|
4 Chapter |
|
Bonds Multiple |
|
||
|
|
Atoms Metal Between |
|
|
|
Compound a |
Crystal |
Virtual |
r(Mo–Mo) (Å) |
Twist |
ref |
|
|
|
|
|
sym. |
sym.b |
Angle (°)c |
|
|
|||
|
|
|
|
|
|
|
|
|||
trans-Mo2(O2CCF3)2(D |
o-OMe |
PhF)2 |
¯ |
D2h |
2.133(2) |
Zero |
154 |
|
|
|
|
1 |
|
|
|||||||
trans-Mo2(O2CCF3)2(PPhpy2)2(ax-O2CCF3)2 |
¯ |
C2v |
2.190(1) |
Zero |
162 |
|
|
|||
1 |
|
|
||||||||
trans-Mo2(O2CCF3)2(Ppy3)2(ax-O2CCF3)2 |
¯ |
C2v |
2.188(1) |
Zero |
162 |
|
|
|||
1 |
|
|
||||||||
cis-Mo2(O2CCF3)2Br2(δ2-Hdpa)·2CH2Cl2 |
1 |
Cs |
2.152(4) |
NR |
64 |
|
|
|||
|
|
|
|
|
|
2.158(4) |
|
|
|
|
|
|
|
|
|
|
|
|
|||
[Mo2(O2CCF3)3(MeNHCH2CH2NHMe)2]O2CCF3 |
1 |
C2v |
2.132(2) |
~0 |
163 |
|
|
|||
[Mo2(O2CCF3)2(S,S-dach)2(CH3CN)2](BF4)2 |
2 |
C2v |
2.155(1) |
~0 |
164 |
|
|
|||
|
|
|
|
|
|
2.154(1) |
|
|
|
|
|
|
|
|
|
|
|
|
|||
Mo2(O2CCF3)2(R,R-dach)2(CH3CN)2]BF4 |
2 |
C2v |
2.153(1) |
~0 |
164 |
|
|
|||
|
|
|
|
|
|
2.153(1) |
|
|
|
|
(NH4)2[Mo2(O2CH)3Cl2]Cl·nH2O |
|
|
2.099(3) |
0 |
113 |
|
|
|||
Rb2[Mo2(O2CH)3Cl2]Cl·nH2O |
|
|
2.106(3) |
0 |
113 |
|
|
|||
trans-Mo2(O2CC6H5)2Br2(PBu |
n |
¯ |
C2h |
2.091(3) |
0 |
165 |
|
|
||
3)2 |
1 |
|
|
|||||||
trans-[Mo2(O2CPh)2(dpmp)2](BF4)2·4CH2Cl2 |
¯ |
C2h |
2.131(4) |
Zero |
140 |
|
|
|||
1 |
|
|
||||||||
trans-[Mo2(O2CPh)2(dpmp-O)2](BF4)2·4CH2Cl2 |
¯ |
C2h |
2.141(2) |
Zero |
140 |
|
|
|||
1 |
|
|
||||||||
(Et4N)2(Mo2(O2CC6H5)2(WS4)2 |
¯ |
C2h |
2.144(1) |
Zero |
166 |
|
|
|||
1 |
|
|
||||||||
Mo2(O2CC6H5)2((NMe3Si)2CC6H5)2 |
2 |
C2v |
2.083(1) |
NR |
167 |
|
|
|||
Mo2(O2CC6H5)2(dppa)2Cl2·2CH3CH2OH |
¯ |
D2h |
2.158(1) |
Zero |
168 |
|
|
|||
1 |
|
|
||||||||
Mo2(O2CC6H5)2(dppa)2Br2·2C7H8 |
¯ |
D2h |
2.176(1) |
Zero |
168 |
|
|
|||
1 |
|
|
||||||||
Mo2(O2CC6H5)2(dppa)2I2·CH3CH2OH·NCCH3 |
1 |
D2h |
2.164(1) |
NR |
168 |
|
Molybdenum |
|||
|
||||||||||
trans-[Mo2(O2CCMe3)2(dpmp)2](BF4)2 |
¯ |
C2h |
2.115(1) |
Zero |
140 |
|
||||
1 |
|
|
||||||||
[Mo2PtCl2(pyphos)2(O2CCMe3)2]2·CH2Cl2 |
1 |
Cs |
2.094(1) |
2.5[5] |
151 |
|
|
|||
[Mo2PtBr2(pyphos)2(O2CCMe3)2]2·CH2Cl2 |
1 |
Cs |
2.096(1) |
3.7[2] |
151 |
|
|
|||
[Mo2PtI2(pyphos)2(O2CCMe3)2]2·CH2Cl2 |
1 |
Cs |
2.102(1) |
3.4[2] |
151 |
|
Compounds |
|||
Mo2(O2CCMe3)3(2-CH2-6-Mepy)·0.5C6H6 |
1 |
Cs |
2.083(1) |
NR |
169 |
Cotton |
||||
[Mo2(O2CCMe3)2(_,_'-bipyrimidine)2](BF4)2·2CH3CN |
1 |
C2v |
2.151(1) |
NR |
169 |
|
|
|||
[Bun4N](Mo2(O2CCMe3)5) |
|
|
1 |
C4v |
2.104(1) |
NR |
170 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
83
|
|
Compound a |
Crystal |
Virtual |
r(Mo–Mo) (Å) |
Twist |
ref |
|
|
sym. |
sym.b |
Angle (°)c |
|||
|
|
|
|
|
|||
Mo2(O2CCH2NH3)2(NCS)4·H2O |
|
1 |
C2v |
2.132(2) |
0 |
171 |
|
|
|
|
1 |
C2v |
2.134(2) |
0 |
|
Mo2(L-isoleucine)2(NCS)4·4.5H2O |
|
1 |
C2v |
2.154(5) |
0 |
171 |
|
|
|
|
1 |
C2v |
2.145(5) |
0 |
|
Mo2(D-valine)(L-valine)(NCS)4·1.5H2O |
1 |
C2v |
2.139(1) |
0 |
85 |
||
Mo2{µ-[(CO)9Co3(µ3-CCO2)]}4[(CO)9Co3(µ3-CCO2H)]2 |
¯ |
D4h |
2.113(1) |
Zero |
172 |
||
1 |
|||||||
Mo2{µ-[(CO)9Co3(µ3-CCO2)]}3(O2CCH3)·C7H8 |
m or 2 |
D4h |
NR |
NR |
172 |
||
Mo2(O2CCH2-p-C6H4OH)4·2THF |
|
¯ |
D4h |
2.097(1) |
Zero |
173 |
|
|
1 |
||||||
Mo2(O2CC(OH)(C6H5)2)4·4THF |
|
|
¯ |
D4h |
2.104(1) |
Zero |
|
|
|
1 |
|
||||
[Mo2(O2CCHF2)2(bpy)2(CH3CN)(BF4)]BF4 |
m |
Cs |
2.143(1) |
0.3 |
174 |
||
Mo6(O2CCHF2)12(bpy)4·4CH3CN |
|
¯ |
C2h |
2.123(2) |
Zero |
|
|
|
1 |
|
|||||
|
|
|
|
|
2.174(1) |
|
|
|
|
|
|
|
|
||
cis-[Mo2(O2CCH2Cl)2(CH3CN)6](BF4)2 |
2 |
C2v |
2.140(2) |
NR |
146 |
||
trans-[Mo2Cl2(O2C(CH2)2CH3)2(µ-dppa)2]·4CH2Cl2 |
¯ |
D2h |
2.172(1) |
Zero |
148 |
||
1 |
|||||||
trans-[Mo2Br2(O2C(CH2)2CH3)2(µ-dppa)2]·4CH2Cl2 |
¯ |
D2h |
2.167(2) |
Zero |
148 |
||
1 |
|||||||
trans-Mo2(O2CCH2CH2CH3)2(D |
o-OMe |
PhF)2 |
¯ |
D2h |
2.109(1) |
Zero |
154 |
|
1 |
||||||
Mo2(O2CCHF2)2(9-EtAH)2(CH3CN)2](BF4)2·2CH3CN |
2 |
C2 |
2.144(2) |
~0 |
175 |
||
9-EtAH = N,N'-9-ethyladenine |
|
|
|
|
|
|
|
a Where more than one set of data is given for any complex this signifies that more than one crystallographically independent molecule is present in the crystal.
bThis is a (partly subjective) estimate of the symmetry that would be possessed by the central unit consisting of the two metal atoms and those portions of the ligands (usually the 8-10 donor atoms) that have an important influence on the electronic structure of the Mo2 unit if it were not subject to any distortion by its neighbors in the crystal. Schoenflies
symbols are used.
c NR means not reported
|
84 |
|
4 Chapter |
|
Bonds Multiple |
|
||
|
|
Atoms Metal Between |
Molybdenum Compounds 85
Cotton
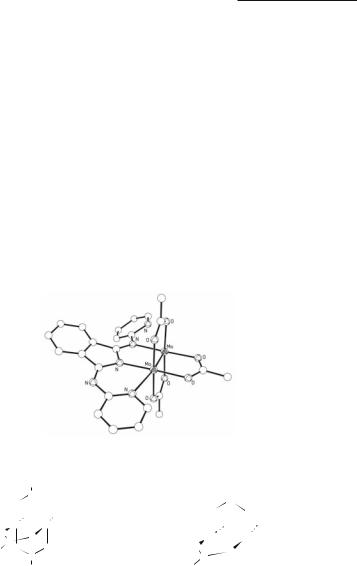
The complex [Mo2(O2CCH3)3(S2CPEt3)(OPEt3)]BF4 is formed upon reacting Mo2(O2CCH3)4 with the zwitterionic ligand S2CPEt3 in THF in the presence of HBF4.114 The structure of the purple/black crystals showed that an axially coordinated Et3PO ligand was present; it is evidently formed by reaction of the S2CPEt3 ligand with water.114 Several complexes of stoichiometry Mo2(O2CCH3)3(BAII), where BAII represents a planar tridentate bis(arylimino)isoindoline ligand, have been prepared from Mo2(O2CCH3)4.115,176
Electronic absorption and 1H NMR spectral measurements have been made on derivatives wherethearylgroupispyridyl,4-methylpyridyl,4-ethylpyridyland4,6-dimethylpyridyl,115,176,177 and the crystal structure of the dark-green pyridyl derivative has been determined (Fig. 4.5). The tridentate nitrogen ligand binds so that one of its pyridyl nitrogen atoms is coordinated at one of the axial sites. Another example is encountered when toluene solutions of Mo2(O2CCF3)4 are treated with Me3SiCl and C2H5CN below 0 °C.160 The orange-red crystals that form have the unusual composition {Mo2(O2CCF3)3Cl(NCC2H5)·Mo2(O2CCF3)2Cl2(NCC2H5)2}; the two molecules jointly comprise the crystallographic asymmetric unit.160 Their structures are represented in 4.3 and 4.4 and the Mo–Mo distances listed in Table 4.2. They pack to form infinite chains of alternating molecules through weak intermolecular Mo···Cl and Mo···O bridges.
Fig. 4.5. The structure of Mo2(O2CCH3)3[bis(pyridylimino)isoindoline].
|
|
R |
|
|
|
R |
|
|
|
|
|
C |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
|
||
O |
O |
|
|
|
|||||
|
|
N |
|
Cl |
O |
O |
|||
|
|
|
|
|
|
N |
|
Cl |
|
Mo |
|
Mo |
|
|
|||||
|
|||||||||
|
|
|
|
|
|
|
|
|
|
O |
O |
Mo |
|
Mo |
||
|
||||||
C O |
O |
O |
|
O |
|
|
R |
C |
C |
|
|
||
|
|
|
|
|
||
|
R |
R Cl |
|
N |
||
|
4.3 |
|
|
4.4 |
|
|
Further substitution of carboxylate groups by halide ions can occur to give anions of stoichiometry [Mo2(O2CR)2X4]2−, as in the cases of (Ph4As)2[Mo2(O2CCH3)2Cl4]·2H2O116,117 and (Bu4N)2[Mo2(O2CCF3)2Br4],58 which are prepared directly from the parent carboxylates upon their reaction with Ph4AsCl and Bu4NBr, respectively. The crystal structures of these complexes have been determined (Table 4.2) and each found to possess a trans arrangement of carboxylate ligands and an eclipsed rotational geometry as shown in 4.5. While the spectroscopic properties of (Ph4As)2[Mo2(O2CCH3)2Cl4], specifically its Raman-active ι(Mo–Mo) mode at 380 cm−1 and β Α β* electronic absorption transition at 20,200 cm−1, lie between the corresponding features in the spectra of Mo2(O2CCH3)4 and K4Mo2Cl8, the Mo–Mo distance is the shortest of the three complexes.

86Multiple Bonds Between Metal Atoms Chapter 4
R2-
C
O X O X
Mo Mo
XX
O O
C
R
4.5
In addition to the nitrile-containing molecules Mo2(O2CCF3)3Cl(NCC2H5) and Mo2(O2CCF3)2Cl2(NCC2H5)2 there are several cationic dimolybdenum(II) species that contain carboxylate and nitrile ligands in the coordination sphere. All those that have been fully characterized contain the [Mo2(O2CCH3)2]2+ moiety, although the number of coordinated nitrile ligands varies; these species can be considered as an intermediate stage in the conversion of Mo2(O2CCH3)4 to [Mo2(NCCH3)8]4+ (see Section 4.3.5).178,179 Treatment of acetonitrile suspensions of Mo2(O2CCH3)4 with stoichiometric amounts of the noncomplexing acids CF3SO3H and HBF4·Et2O has been described180 as forming materials of composition [Mo2(O2CCH3)2(NCCH3)4](SO3CF3)2 and [Mo2(O2CCH3)2(NCCH3)5](BF3OH)2, respectively, that were characterized by spectroscopic means. A recipe similar to that used to prepare the first of these complexes was later found to give the crystalline complex Mo2(O2CCH3)2(NCCH3)4(O3SCF3)2.118 A crystal structure determination revealed118 a cis-arrangement of acetate groups, and weakly axially bound triflate anions. The use of (Et3O)BF4 in place of HBF4·Et2O gave the hexakis(acetonitrile) complex cis-[Mo2(O2CCH3)2(NCCH3)6](BF4)2, whose structure resembles that of the triflate derivative except that additional acetonitrile ligands have replaced the [CF3CO2]− anions in the axial sites,118 as shown in Fig. 4.6. There is a large discrepancy between the Mo–N distances of the equatorially and axially bound nitrile ligands (c. 2.15 Å versus 2.70 Å).118 The same complex is also formed when (Et3O)BF4 is replaced by (Me3O)BF4,181,182 a procedure that can be adapted to give the formato complex [Mo2(O2CH)2(NCCH3)4](BF4)2.182 The isolation of only a tetrakis complex in the latter case (albeit impure) indicates that the axially bound nitriles are very labile, and in accord with this expectation the NMR spectra of the acetonitrile complex show that the equatorial and axial CH3CN ligands interchange rapidly.182 This has also been shown to be the case with [Mo2(O2CBut)2(NCCH3)6]2+, a species which also undergoes a rapid reaction with Mo2(O2CBut)4 according to the following equilibrium:183
|
|
CH3CN |
||
Mo2(O2CBut)4 + [Mo2(O2CBut)2]2+ |
|
|
|
2[Mo2(O2CBut)3]+ |
|
|
|||
|
|
|
||
The lability of the acetonitrile ligands of cis-[Mo2(O2CCH3)2(NCCH3)6](BF4)2 has been shown by the reactions of this complex with the Me2PCH2CH2PMe2 (dmpe) and with the chiral ligand (2S,3S)-bis(diphenylphosphino)butane(S,S-dppb) to give trans-[Mo2(O2CCH3)2(µ- dmpe)2](BF4)2 and [Mo2(O2CCH3)2(S,S-dppb)(NCCH3)2](BF4)2, respectively.119 The X-ray crystal structure of the former complex shows that the dmpe ligands bridge the Mo atoms so as to maintain a rigorously eclipsed rotational geometry. The rings adopt a half chair conformation, like that of cyclohexane, but they possess opposite chirality so as to give the complex an overall D2h symmetry. This geometry is retained in solution.119 Reaction of [Mo2(O2CCH3)2(CH3CN)6]2+ with 1,4,7-trithiacyclononane (TTCN) affords the compounds [(TTCN)Mo(µ-O2CCH3)2Mo(N CCH3)3](BF4)2 and [(TTCN)Mo(µ-O2CCH3)2Mo(TTCN)](BF4)2 which are formed in stepwise fashion. The first of these reacts with KX in aqueous solution to form blue species of stoichiometry (TTCN)Mo(µ-O2CCH3)2MoX2 (X = Cl, Br, SCN or OCN).184

Molybdenum Compounds 87
Cotton
Fig. 4.6. The cation in cis-[Mo2(O2CCH3)2(CH3CN)4(ax-CH3CN)2](BF4)2.
An extensive series of dimolybdenum(II) carboxylate complexes are those of stoichiometry Mo2(O2CR)2X2(PR3)2, where X represents an alkyl, amido, siloxy or halide ligand. The first alkyl derivatives to be isolated were obtained by the reaction of Mg(CH2SiMe3)2 and Mg(CH2CMe3)2 with mixtures of Mo2(O2CCH3)4 and PMe3 with a MgR2: Mo2(O2CCH3)4 reaction stoichiometry of 2:1.185,186 The benzyl and p-methyl benzyl complexes of this type were prepared by a similar procedure,121 as were the pivalate complexes Mo2(O2CCMe3)2R2(PMe2Et)2 (R = CH2SiMe3 or CH2CMe3).122 X-ray crystal structure determinations on Mo2(O2CCH3)2(CH2SiMe3)2(PMe3)2120 and Mo2(O2CCH3)2(CH2Ph-p-Me)2(PMe3)2121 have shown that these complexes possess the centrosymmetric trans structure represented in 4.6. The P–Mo–C angles of c. 142° are probably a consequence of the steric demands of the alkyl and phosphine ligands. The phenyl and 4-fluoro- phenyl complexes of stoichiometry Mo2(O2CCH3)R3(PMe3)3, where R = Ph or 4-F-Ph, are the products of the reaction between Mo2(O2CCH3)4 and the magnesium diaryl in diethyl ether containing an excess of trimethylphosphine.187 In the absence of phosphine, decomposition has been found to occur. An unsymmetrical structure is clearly in order, and this is supported by NMR spectroscopy.187
R
C
OO
X 
 PR3
PR3
Mo Mo
R3P X
O O
C
R
4.6
The acetate Mo2(O2CCH3)4 reacts in diethyl ether with LiN(SiMe3)2, LiN(SiMe2H)2 or LiN(SiMe3)(Me) in the presence of tertiary phosphines (PMe3, PEt3 or PMe2Ph) to give red pentane-soluble complexes of the type Mo2(O2CCH3)2(NR2)2(PR3)2. Infrared and NMR (1H, 13C and 31P) spectroscopy have been used188 to demonstrate that the particular isomer formed is dependent upon the nature of the NR2 ligand. With [N(SiMe2H)2]− the structure is similar to 4.6, but the other two silylamido ligands apparently give the isomer in which the pairs of PR3 and silylamido groups are trans to each other on different molybdenum atoms. The analogous pivalate complex Mo2(O2CCMe3)4 has been reported188 to react in a similar fashion, with the exception that the bis(trimethylsilyl)amido complexes are of stoichiometry Mo2(O2CCMe3)3- [N(SiMe3)2]2](PR3) (PR3 = PMe3, PEt3 or PMe2Ph). The compounds Mo2(O2CCF3)2[N(SiMe3)2]2- (PMe3)2188 and Mo2(O2CCMe3)2[N(SiMe2H)2]2(PMe2Et)2122 have also been described.
The preparation of the siloxy complexes, Mo2(O2CCMe3)2(OSiMe3)2(PR3)2 (PR3 = PMe3 or PMe2Et) from the reaction of Mo2(O2CCMe3)4, LiOSiMe3, and PR3 in diethyl ether, has been re-

88Multiple Bonds Between Metal Atoms Chapter 4
ported.122 The crystal structure of the acetate complex Mo2(O2CCH3)2(OSiMe3)2(PMe3)2 shows122 the geometry to be as in 4.6; the Mo–Mo distance of 2.114(1) Å is similar to the distances reported for the structurally characterized alkyl derivatives (Table 4.2) and the P–Mo–O(siloxyl) angle (149°) is slightly larger than the P–Mo–C angles of these same two alkyl complexes.
Several procedures that have been utilized to prepare halide complexes of the type Mo2(O2CR)2X2(PR'3)2 R = alkyl or aryl; X = Cl or Br; PR'3 = monodentate phosphine) are as follows:
Mo2(O2CCH3)4 + AlCl3 + PPh3 Α Mo2(O2CCH3)2Cl2(PPh3)2189
Mo2(O2CR)4 + Me3SiX + PR'3 Α Mo2(O2CR)2X2(PR'3)2123-125,190
(R = CH3 or CMe3; X = Cl or Br; R' = Me, Et, Bun or Ph)
Mo2X4(PR'3)4 + RCO2H Α Mo2(O2CR)2X2(PR'3)2107,125
(X = Cl or Br; R' = Et or Bun; R = CMe3, Ph or 2,4,6-Me3Ph)
While molecules of this type were first synthesized by San Filippo and coworkers107,165 with the use of the third of these methods, the second method has subsequently become the most popular one. It is adaptable to a range of Me3SiX reagents and can also be used to prepare compounds of the type Mo2Cl4L4 (see Section 4.3.4) by the complete expulsion of all the carboxylate ligands.123 The THF complex Mo2(O2CCH3)2Cl2(THF)2 has also been prepared by this same type of procedure. X-ray structure determinations have been carried out on several of these derivatives (Table 4.2) and, with one exception, they have been found125 to possess the centrosymmetric trans structure 4.6, like that of their alkyl120,121 and siloxy122 analogs. The exception is Mo2(O2CCMe3)2Cl2(PEt3)2 which has been isolated and structurally characterized in both its trans (4.6) and cis (4.7) isomeric forms.125 The isomers designated as _- and `-Mo2(O2CCH3)2X2(PEt3)2 by Green et al.123 probably correspond to structures 4.7 and 4.6, respectively.
R
C
OO R
 O
O C
C
 O
O
Mo Mo
Mo
R3P X
XPR3
4.7
The lability of the PPh3 ligand of trans-Mo2(O2CCH3)2Cl2(PPh3)2 has been demonstrated by the conversion of this complex to the related PEt3 and PBun3 derivatives.123 These reactions proceed by a stepwise dissociative mechanism as shown122 by studies of the reactions of Mo2(O2CCMe3)2X2(PMe2Et)2 X = CH2SiMe3, CH2CMe3, CH3, Cl, Br, I, N(SiMe2H)2 or OSiMe3) with PMe3 at low temperatures to give Mo2(O2CCMe3)2X2(PMe2Et)(PMe3). From the magnitude of the 3JPP coupling constants, the structural trans effect was deduced to be alkyl > halide > amide > siloxy, an order that mirrors the kinetic trans effect.122
The aforementioned phosphine lability is further shown by the reaction of Mo2(O2CCH3)2Cl2(PPh3)2 with the bidentate phosphine Ph2PCH2PPh2 (dppm) in THF to give red-violet Mo2(O2CCH3)2Cl2(µ-dppm) when short reaction times are used.190 As an al-
