
Multiple Bonds Between Metal Atoms / 04-Molybdenum Compounds
.pdf
Molybdenum Compounds 139
Cotton
Fig. 4.22. The structure of Mo2[F2PN(CH3)PF2]4Cl2. This chiral molecule has idealized D4 symmetry.
Oxidation of Mo2(O2CR)4 (R = C2H5, CMe3 or Ph) in 1,2-dichloroethane by iodine was to afford [Mo2(O2CR)4]I3 products which have relatively narrow EPR signals (g = 1.93 ± 0.01). In another early report of a chemical oxidation, CCl4 in CH2Cl2 oxidized
(Ph4P)2[Mo2(O2CPh)4Cl2] to give (Ph4P)2[Mo2(O2CPh)4Cl4], but here the paddlewheel structure was changed to that of an edge-sharing bioctahedron.512
It was not until relatively recently that compounds containing [Mo2(O2CR)4]+ ions were actually prepared and the ions studied in more detail.513 The three compounds prepared were [Mo2(2,4,6-Pri3C6H2)4]X (X = BF4, PF6) and [Mo2(O2CCMe3)4]PF6. All have Mo–Mo distances in the range 2.136(1)-2.151(1) Å, which may be compared with the Mo–Mo distances of the neutral Mo2(O2CR)4 compounds of c. 2.09 Å. There has never been any indication that [Mo2(O2CR)4]2+ ions can be obtained.
The earliest isolated and well-characterized example of an Mo25+ compound was the [Mo2(SO2)4]3− ion, which was discovered196 by chance in 1973. Attempts to recrystallize K4[Mo2(SO)4)4]·2H2O gave small amounts of the oxidized species. It was then found that it can be obtained in good yield by using an air stream to oxidize a solution of K4[Mo2(SO4)4]·2H2O in 2 M H2SO4 until the color changes from red to pale blue. It is also possible to form [Mo2(SO4)4]3− from [Mo2(SO4)4]4− by irradiating the former in 5 M H2SO4 with ultraviolet light (254 nm).206,205 The Mo–Mo distance in [Mo2(SO4)4]3− is 2.167(1) Å as compared to 2.111(1) Å in [Mo2(SO4)4]4−.
Further oxidation of the [Mo2(SO4)4]3− ion to an isolable compound of the triply-bonded (μ2/4) [Mo2(SO4)4]2− ion has not been accomplished, but the similar [Mo2(HPO4)4]2− ion can be made simply by dissolving K4Mo2Cl8·2H2O in aqueous 2 M H3PO4 and exposing the solution to air for 24 h. When large cations such as Cs+ and pyH+ are present, purple crystalline products are obtained.197 An electrochemical study216 of the [Mo2(HPO4)4]2− ion showed that reductions to the 3− ans 4− ions require potentials of −0.25 and −0.67 V versus SCE in 2 M H3PO4 solution.
The ability of bridging ligands such as SO42− and HPO42− to stabilize Mo25+ and Mo26+ cores better than uninegative bridging ligands such as the carboxylate ions, is essentially electrostatic in nature: the large amount of negative charge surrounding the Mo2n+ core makes higher values of n more attainable and stable.
An interesting sequel to the story of the sulfato and phosphato complexes of Mo25+ and Mo26+ began with a report514 in 1989 of compounds alleged to contain the Mo24+ core com-
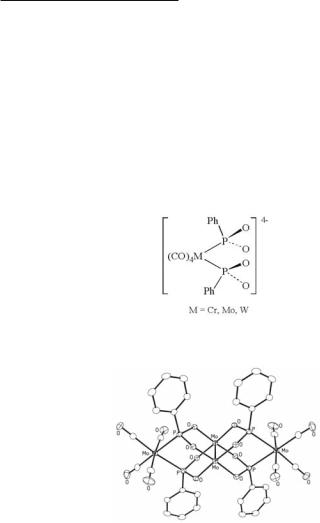
140Multiple Bonds Between Metal Atoms Chapter 4
plexed by two ligands, L, of the type 4.31. The complex anions, [Mo2L2]4−, were accompanied by only two +1 cations, but the presence, at an unstated location, of two H+ ions was postulated in the one case where a structure was reported.514 Moreover, the Mo–Mo distance was found to be 2.186(2) Å. In 2002 the suspicious character of these compounds was cleared up.515 An abundance of evidence shows that they are complexes of the Mo26+ core. The highly oxidized core is stabilized by the total of eight negative charges, the Mo–Mo distance is consistent with a bond order of three, and the postulated protons are not present. A drawing of the [Mo2L2]2− anion in one of the four compounds studied is presented in Fig. 4.23. This structure (and the absence of any other cations, protons or otherwise) was exhaustively characterized by crystallography employing four polymorphs of [NBun4]2Mo2L4 where L is the anion 4.31 with M = Mo. All of the pertinent data are listed in Table 4.14.
4.31
Fig. 4.23. The dianion in (NBun4)2{Mo2[Mo2(CO)4(PhPO2)2]2}.
Table 4.14. Structural data for (NBun4)2Mo2[Mo(CO)4(PPhO2)2]2
Space group |
Mo–Mo distance |
Remarks |
P21/n |
2.178(8) |
no solvent, neutron diffraction |
P21/n |
2.190(1) |
no solvent, X-ray diffraction |
P21/n |
2.223(1) |
axial THF molecules |
¯ |
2.193(1) |
CH2Cl2 present |
P1 |
||
Pbca |
2.187(1) |
no solvent |
The fact that the ligand 4.31 has the ability to stabilize the Mo26+ core, however, does not entirely account for the formation of the [Mo2L2]2− ions, since the preparations all begin with Mo2(O2CCH3)4 or another Mo24+ compound and no recognized oxidizing agent is used. The explanation is that the solvent, CH2Cl2 or C2H5OH, in which the reaction is carried out oxidizes

Molybdenum Compounds 141
Cotton
the initially formed [Mo2L2]4− complex. In the non-oxidizing solvent THF a reversible wave corresponding to the process
[Mo2L2]2- |
|
+e |
[Mo2L2] |
3- |
|
|
|
|
|
||
-e |
|
||||
was observed at −1.54 V vs Ag/AgCl, showing that even the mildest oxidizing agents can take Mo24+ to Mo26+ when it is coordinated by two 4.31 ions. The use of the 4.31 type ligands represents the extreme known limit of employing highly charged ionic ligands to stabilize highly charged M2n+ cores. These ligands have not yet been used with any cores other than Mo26+.
In addition to this “ionic ligand” approach, there is also a “covalent ligand” or “noninnocent ligand” approach to the stabilization of highly oxidized M2n+ cores. This approach, which also originated with Mo2n+ chemistry (but has been extended to W2n+, Re2n+ and several other metals) is based on guanidinate type ligands, 4.32. The first two examples of neutral paddlewheel complexes504,505,516 with guanidinate bridges are those with L = hpp (4.33) and 1,2,3- triphenylguanidinate (4.34) as ligands. In both cases it was immediately noted that oxidation occurs readily and the oxidation products can be easily isolated and characterized. From 4.33 Mo2(hpp)4(BF4)2 was obtained517 and shown to have a Mo–Mo distance of 2.142(2) Å, which is 0.075 Å longer than that in Mo2(hpp)4, as a result of the combined effects of two β electrons being lost and the charges on the Mo2 unit increasing from +4 to +6. Similarly, for the oxidation of Mo2(1,2,3-triphenylguanidinate)4 to the corresponding Mo25+ compound, the Mo–Mo distance increases from 2.084(1) to 2.119(1) Å.
|
|
|
|
|
|
N |
|
|
|
N |
|
|
|
|
|
||||
|
|
|
|
|
N |
N 4 |
|||
|
|
|
|
|
|
|
|
|
|
C |
|
|
|
|
|
||||
|
|
|
|
|
Mo |
Mo |
|||
N |
N |
||||||||
|
|
|
|
||||||
4.32 |
|
4.33 |
4.34 |
||||||
The Mo2(hpp)4 molecule is so easily oxidized that it cannot dissolve in dichloromethane without undergoing the following reactions:518
In Mo2(hpp)4Cl the Mo–Mo distance is 2.128(1) Å and in Mo2(hpp)4Cl2 it is 2.174(1) Å.
The electrochemistry of the two Mo2(guanidinate)4 molecules is truly remarkable when compared to that of all other Mo2 paddlewheel compounds. Whereas the most easily oxidized Mo2(DArF)4 molecule (Ar = p-anisyl) has an Mo24+/Mo25+ potential of +142 mV, for the Mo2(hpp)4 molecule the corresponding oxidation occurs at −1271 mV, and the [Mo2(hpp)4]+/ [Mo2(hpp)4]2+ potential is −444 mV. For Mo2[(PhN)2CNHPh]4, the corresponding potentials are −50 mV and +850 mV. The basis for the extraordinary ability of guanidinate ligands to stabilize the higher oxidation states of M2n+ cores in general is still under study.
An important study of the influence of the ligands on the Mo24+/Mo25+ potential was reported in 1995 by Ren et al.499,500 They found that for a series of Mo2(DArF)4 compounds with various Ar groups the voltage varied systematically with the Hammett μ constant for the sub-

142Multiple Bonds Between Metal Atoms Chapter 4
stituents in the XC6H4 aryl groups. This is shown in Fig. 4.24 where the potentials have been corrected for errors in the original data that resulted from contamination by H2O.501
The most extensive electrochemical studies have been carried out on compounds with pairs of Mo24+ cores linked by dicarboxylic anions, diamidato anions, SO42− ions, etc. These results are all presented in Sections 4.5.1 to 4.5.7.
Fig. 4.24. The linear relationship of the oxidation potentials of Mo2(DArF)4 compounds to the Hammett μ-parameters of the aryl groups.
4.4.3 Hydrides and organometallics
There is a curious phosphine hydride molecule, (Me3P)3HMo(µ-H)2MoH(PMe3)3, which was prepared by reaction of Mo2(O2CCH3)4 with Na/Hg in THF in the presence of excess PMe3 under 3 atm pressure of hydrogen.519,520 It is a centrosymmetric edge-sharing bioctahedron, but is of interest here because the Mo–Mo distance within the Mo2(µ-H)2 unit is very short, viz., 2.194(3) Å. The 1H NMR spectrum is consistent with retention of this structure in solution. It is pyrophoric and reacts rapidly with alkyl halides, olefins, acetylenes, CO and H2S, but no defined products were isolated.520
Like the isoelectronic species [Re2(CH3)8]2− and [Cr2(CH3)8]4−, the octamethyldimolybdate anion [Mo2(CH3)8]4− has been prepared and successfully characterized.292 The pyrophoric lithium salts Li4Mo2(CH3)8·4L, L = diethyl ether, tetrahydrofuran or 1,4-dioxane, were prepared292 by reacting Mo2(O2CCH3)5 with diethyl ether solutions of MeLi followed by recrystallization from the appropriate ether solvent. The structure of the anion, as determined in the THF solvate, is that of the familiar centrosymmetric, eclipsed Mo2L8 unit of D4h symmetry, with a short Mo–Mo distance, 2.148(2) Å. The ether molecules do not bind axially to the [Mo2(CH3)8]4− ions, probably reflecting the low electrophilicity of this anion. An alternative route to Li4Mo2(CH3)8·4THF involves the reaction of methyllithium with the mononuclear starting material MoCl3(THF)3 in diethyl ether at −30 °C,521 although at the time this reaction was first reported it was not recognized as leading to a quadruply-bonded dimolybdenum complex.
Li4Mo2(CH3)8·4ether complexes are thermally stable at room temperature in the absence of oxygen and moisture. They react rapidly with acetic acid-acetic anhydride at −78 °C to regenerate Mo2(O2CCH3)4. The ether solvent molecules are replaceable by, for example, ammonia, pyridine, acetonitrile, acetamide, and hexamethylphosphoramide, but the products of many other reactions have not been identified owing to their instability and/or insolubility.
In the presence of trimethylphosphine, Mo2(O2CCH3)4 reacts with Mg(CH3)2 at 25 °C to afford the blue, air-stable, and volatile complex Mo2(CH3)4(PMe3)4. While dimethyl-

Molybdenum Compounds 143
Cotton
phenylphosphine forms the analogous dimer Mo2(CH3)4(PMe2Ph)4, complexes with methyldiphenylphosphine, triphenylphosphine, and trimethylphosphite could not be obtained.186 The complex Mo2(CH3)4(PEt3)4 has been prepared522 from Mo2(O2CCMe3)4, MeMgCl and PEt3 in diethyl ether, followed by crystallization at −10 °C. This complex undergoes rapid exchange with excess PMe2Ph or PMe3 in toluene solution to give Mo2(CH3)4(PMe2Ph)4 or Mo2(CH3)4(PMe3)4.522 It has been shown400 subsequently that the preparation of Mo2(CH3)4(PR3)4 from the reaction between Mg(CH3)2, Mo2(O2CCH3)4 and PR3 requires rigorously chloride-free conditions if contamination by Mo2Cl4(PMe3)4 is to be avoided. This can be accomplished by the use of Mg(CH3)2 prepared from Hg(CH3)2. The preparative method that uses the Grignard reagent CH3MgCl522 also introduces chloride contaminants.400 Indeed, the reaction between Mo2(O2CCH3)4, PhCH2MgCl and PMe3 in THF at −78 °C affords the mixed chloride-alkyl Mo2Cl3(CH2Ph)(PMe3)4.400
The NMR spectra of the complexes Mo2(CH3)4(PR3)4 are similar,186,522 and are in accord with a structure like that found for their halide analogs. These structural conclusions have been confirmed400 by X-ray crystal structure determinations on Mo2(CH3)4(PMe3)4 and Mo2(CH3)4(PMe2Ph)4, as well as on Mo2Cl3(CH2Ph)(PMe3)4. An earlier report522 on the structure of Mo2(CH3)4(PMe3)4 has been shown400 to be vitiated by serious contamination from chlo- ride-containing impurities.
The phosphine-exchange reactions of Mo2(CH3)4(PEt3)4 with PMe2Ph and PMe3 in toluene have been shown by NMR spectroscopy to occur in a stepwise fashion through a dissociative mechanism.400 The driving force for these reactions is believed to be the relief of steric congestion as PEt3 is replaced by the smaller PMe2Ph or PMe3 ligands. In further studies of their reactivity, it has been shown that the Mo2(CH3)4(PR3)4 compounds (PR3 = PMe3, PEt3 or PMe2Ph) react with CO (18 atm) at room temperature in benzene to give acetone and compounds of the type Mo(CO)6−x(PR3)x (x = 0-3). The reaction rates are in the order PEt3 >> PMe2Ph > PMe3, suggesting that the rate determining step involves PR3 dissociation.
The reaction of an excess of Mg(CH2SiMe3)2 with a mixture of Mo2(O2CCH3)4 and PMe3 leads to an air-sensitive compound of formula Mo2(CH2SiMe3)2[(CH2)2SiMe2](PMe3)3 whose structure is represented in Fig. 4.25.186 A related complex containing trimethylphosphite has also been isolated.186 This unusual molecule has a metal–metal bond distance (c. 2.16 Å) that seems at first sight to be in accord with a quadruple bond. It contains two electronically different metal atoms that may be represented formally as MoI and MoIII, a situation that would be compatible with a Mo–Mo quadruple bond that included a dative component, or a triple bond. This may be another example like Mo2(OPri)4(dmpe)2, of an intramolecular disproportionation reaction. In this complex the two metal atoms are bridged by a (CH2)2SiMe2 group that could form by the elimination of a α-hydrogen from a terminal MoCH2SiMe2 unit.
Mixed alkyl phosphine complexes of Mo24+ may also be obtained through the reactions of trimethylphosphine with the triply-bonded dimolybdenum(III) complexes Mo2Br2(CH2CMe3)4 and Mo2Br2(CH2SiMe3)4.523 The neopentyl derivative affords Mo2(CH2CMe3)4(PMe3)4 while Mo2Br2(CH2SiMe3)4 yields Mo2Br2(CH2SiMe3)2(PMe3)4,524 but both are the consequence of reductive eliminations.
Several alkynyl-substituted, quadruply bonded complexes of the type Mo2(δ1-CCR)4(PMe3)4 (R = CHMe2, CMe3, SiMe3 or Ph) have been prepared by the reaction of LiCCR with Mo2Cl4(PMe3)4 in diethyl ether-dimethoxyethane mixtures.507 Their electronic absorption and resonance Raman spectra show characteristics that are manifestations of the conjugation between the [β,β*] orbitals of the Mo24+ core and the [/, /*] orbitals of the CCR ligands.

144Multiple Bonds Between Metal Atoms Chapter 4
Fig. 4.25. The structure of Mo2(CH2SiMe3)2[(CH3)2SiMe2](PMe3)3.
Two other important organometallic derivatives are the allyl and cyclooctatetraene (COT) compounds Mo2(C3H5)4 and Mo2(COT)3. The former has been prepared either by reaction of MoCl5 with allylmagnesium chloride in diethyl ether,525,526 or by treating Mo2(O2CCH3)4 with four equivalents of allyllithium or allylmagnesium bromide.292 The cyclooctatetraene derivative has been obtained by a procedure that involves reduction of a mixture of MoCl5 in THF by K2C8H8 to give the black crystalline complex Mo2(COT)3.527 Both molecules may be construed as possessing Mo–Mo quadruple bonds but the rather low symmetry of these molecules and the complex ligand array has not encouraged a detailed treatment of the bonding in either case.
The use of Mo2(δ3-C3H5)4 as a precursor complex for the preparation of active catalysts containing Mo2 species on alumina or silica is well documented.528-533 The catalysts show high activities for ethylene or 1,3-butadiene hydrogenation, propene metathesis, and other important organic reactions. The thermodynamic and kinetic stability of the isomers of Mo2(δ3-C3H5)4 and its methylallyl analog have been studied534 as well as their Lewis base catalyzed isomerization. The reactions of `-diketones with Mo2(δ3-C3H5)4 afford Mo2(µ2-δ3-C3H5)2(δ2-L)2, where L = acac, tfac and hfac,535 while reaction of Mo2(δ3-C3H5)4 with carbon monoxide induces the reductive elimination of two pairs of allylic ligands and the formation of mononuclear Mo(δ4- 1,5-hexadiene)(CO)4.536
The orange, diamagnetic phosphine ylid compound, Mo2[(CH2)2PMe2]4, can be obtained by both of the following reactions:537
MoCl3(THF)3 + Li[(CH2)2PMe2] Α Mo2[(CH2)2PMe2]4
Li4[Mo2Me8] + 4Me4PCl Α Mo2[(CH2)2PMe2]4 + 8CH4 + 4LiCl
In the first one the oxidation product from the Li[(CH2)2PMe2] was not identified. The second reaction illustrates the utility of Li4[Mo2Me8] as a synthon, although it has not been widely exploited as such. The Mo–Mo distance in the tetrakis ylid molecule is 2.082(2) Å; the molecule has an inversion center and virtual C4h symmetry.538
There are two compounds187,539,540 in which the Mo24+ unit is bridged by four 2-methoxy- phenyl or 2,6-dimethoxyphenyl groups (4.35). There is an exceptionally short quadruple bond in the Mo2(2,6-dimethoxyphenyl)4 molecule,540 namely, 2.064(1) Å, the structure of which is shown in Fig. 4.26.

Molybdenum Compounds 145
Cotton
Me
O |
C |
X 2 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
Mo |
|
Mo |
X = H, OMe |
||
|
|||||
|
|||||
X C O
Me
2
4.35
Fig. 4.26. The structure of the Mo2(2,6-dimethoxyphenyl)4 molecule.
4.4.4 Heteronuclear Mo–M compounds
Apart from a few compounds containing MoRe, MoRu and MoOs bonds (vide infra) this class of compounds is limited to those with MoCr and MoW bonds. All the known structures are listed in Table 4.15. Incidentally, no CrW compound has been reported.
Table 4.15. Structures of Mo–M compounds
Formula |
Mo–M |
ref. |
MoCr(O2CCH3)4 |
2.050(1) |
304 |
MoW(O2CCMe3)4 |
2.080(1) |
324 |
MoW(O2CCMe3)4I |
2.194(2) |
541 |
MoW(mhp)4 |
2.091(1) |
246 |
MoWCl4(PMe3)4 |
2.209(2) |
439 |
(PMe3)2Cl2MoWCl2(PMePh2)2 |
2.207(1) |
439 |
MoWCl4(PMePh2)4 |
2.210(4) |
439 |
|
2.207(4) |
439 |
MoWCl4(PMe2Ph)4 |
2.208(1) |
542 |
MoWBr4(PMe2Ph)4 |
2.209(1) |
543 |
(PMe2Ph)2Cl2MoWCl2(PMe2Ph)(PPh3) |
2.216(1) |
542 |
(PMe2Ph)(PPh3)Cl2MoWCl2(PMe2Ph)2 |
|
|
`-MoWCl4(dppm)2 |
2.211(1) |
544 |
`-MoWCl4(dppe)2 |
2.243(1) |
544 |

146Multiple Bonds Between Metal Atoms Chapter 4
Formula |
Mo–M |
ref. |
`-MoWCl4(dmpm)2 |
2.193(2) |
545 |
[(OEP)MoRu(TPP)]PF6 |
2.181(2) |
546 |
[(TPP)MoRu(OEP)]PF6 |
2.211(2) |
547 |
[(OEP)MoOs(TPP)]PF6 |
2.238(3) |
548 |
[(TPP)MoRe(OEP)]PF6 |
2.235(1) |
547 |
The two tetraacetato molecules, CrMo(O2CCH3)4 and MoW(O2CCH3)4 are well characterized. The former was made in 30% yield by addition of Mo(CO)6, dissolved in a mixture of acetic acid, acetic anhydride and CH2Cl2, to a refluxing solution of Cr2(O2CCH3)4(H2O)2 in acetic acid/acetic anhydride.304 It can also be obtained by reaction of Mo2Br4(CO)8 with an excess of CrCl2 in acetic acid.549 Yellow, volatile CrMo(O2CCH3)4 is isomorphous with Mo2(O2CCH3)4 and the Cr–Mo distance, 2.050(1) Å, is between those in Mo2(O2CCH3)4 and gaseous Cr2(O2CCH3)4. It displays a parent ion peak in the mass spectrum and has a Raman-ac- tive Cr–Mo stretching mode at 394 cm−1. Impure CrMo(O2CCF3)4 has been obtained by treatment of CrMo(O2CCH3)4 with CF3COOH.304
The yellow, crystalline MoW(O2CCMe3)4 was made by refluxing a 1:3 mixture of Mo(CO)6 and W(CO)6 with pivalic acid in o−dichlorobenzene.11,541 The initial product consists of about 70% MoW(O2CCMe3)4 and 30% Mo2(O2CCMe3)4. The separation and purification of the pure MoW compound was accomplished by oxidizing it to MoW(O2CCMe3)4I, separating this, then reducing it back to MoW(O2CCMe3)4 by Zn in acetonitrile. The compound shows a parent ion peak in the mass spectrum and it has an Mo–W distance324 of 2.080(1) Å, which is but slightly shorter than that in Mo2(O2CCMe3)4, 2.088(2) Å.35 In the compound MoW(O2CCMe3)4I,541 the iodine atom is bonded to the tungsten atom and the structure is ordered, with a Mo–W distance of 2.194(2) Å.
Similarly, MoW(mhp)4 is formed246 by refluxing a 3:2 mixture of Mo(CO)6 and W(CO)6 with Hmhp in a mixture of diglyme/heptane. Like its pivalate analog, MoW(mhp)4 can be purified, and thereby freed of any Mo2(mhp)4 contaminant, by oxidation with iodine to [MoW(mhp)4]+ followed by reduction with zinc amalgam.246 The dichloromethane solvate MoW(mhp)4·CH2Cl2 is isomorphous with the other members of the mhp series, and the Mo–W distance of 2.091 Å falls between the corresponding Mo–Mo and W–W distances, but is shorter by 0.022(2) Å than the average of the latter two. A very small amount of impure CrMo(mhp)4 has also been obtained, both by a procedure analogous to that used to prepare MoW(mhp)4247 and also upon treating CrMo(O2CCH3)4 with Na(mhp) in ethanol.
An elegant, efficient and general route to certain complexes of the MoW4+ core was first reported in 1984440 and in more detail in 1987.439 It takes advantage of the reactivity of the phosphine arene complexes 4.36 and 4.37, with WCl4, as illustrated in the following equation:
(δ6-PhPMe2)Mo(PPhMe2)3 + WCl4(PPh3)2 Α (PPhMe2)2Cl2MoWCl2(PPhMe2)2 + 2PPh3
With this approach the two compounds MoWCl4(PMe2Ph)4 and MoWCl4(PMePh2)4 were obtained. Small amounts of the Mo2Cl4(PR3)4 compounds were also formed.

 PPhMe
PPhMe
Mo
Ph2MeP PMePh2
PMePh2
4.36 |
4.37 |

Molybdenum Compounds 147
Cotton
By taking advantage of the ability of the basic PMePh2, the following reactions were
smaller, more basic PMe3 to replace the larger, less accomplished:
|
|
2 |
|
|
n |
= |
|
|
|
|
|
MoWCl4(PMePh2)4 + nPMe3 |
|
n = |
4 |
MoWCl4(PMe3)2(PMePh2)2
or
MoWCl4(PMe3)4
In general these MoW compounds crystallize so the two kinds of metal atoms are disordered over the two metal atom sites. This leads to a situation where the reported uncertainties in the bond lengths and angles undoubtedly underestimate the actual ones. The structure of the mixed phosphine complex is an exception to this because the PMe3 ligands are both on the molybdenum atom and the PMePh2 ligands are both on the tungsten atom, and the molecules are ordered. This structure is shown in Fig. 4.27.
Fig. 4.27. The structure of (PMe3)2Cl2MoWCl2(PMePh2)2.
In a modification542 of the above procedure, PPh3 was included in the reaction of (δ6-PhPMe2)Mo(PMe2Ph)3 with WCl4(PPh3)2. This led to the formation of a mixture of (PMe2 Ph)2Cl2MoWCl2(PMe2Ph)(PPh3) and (PMe2Ph)(PPh3)Cl2MoW(PMe2Ph)2, which co-crystallize with the Mo and W atoms disordered. The compound MoWBr4(PMe2Ph)4 has been made by reaction of (δ6-PhPMe2)Mo(PMe2Ph)3 with WBr5.543
It should be noted that the type of reaction used to make the MoWCl4(PMenPh3−n)4 molecules is also effective for making the Mo2X4(PMenPh3−n)4 molecules if MoCl4(THF)2 is used in place of WCl4(PPh3)2. However, as already noted in Section 4.3.4 the homonuclear molecules can be obtained in more conventional ways.
Extensive studies have been made of MoWCl4(diphos)2 compounds,544,545 which were obtained by the action of the diphosphines on MoWCl4(PMePh2)4. The diphosphines employed were dppm, dppe, dmpm and dmpe. As with the homonuclear Mo2X4(diphos)2 compounds, two isomeric types, _ and `, occur. No X-ray structures of _-MoWCl4(diphos)2 molecules have been reported but there is NMR evidence for their existence.
In general the MoW compounds resemble their homonuclear analogs. The polarity of the MoW bond is probably small and has no significant effect on the properties of the compounds. An electronic absorption band in the region of 630-650 nm has been reported for several of them and is presumed to be due to a β Α β* transition. Several of the MoWCl4(PR3)4 compounds appear to undergo oxidation at c. +0.45 V versus Ag/AgCl, but there is no report of any such oxidation having been carried out chemically. The photoelectron spectrum550 of

148Multiple Bonds Between Metal Atoms Chapter 4
MoWCl4(PMe3)4 shows three resolved peaks that have been assigned to β, / and μ electrons, in increasing order of energy.
There are only a few reported compounds containing MM' multiple bonds between metal atoms from different groups of the periodic table; all of them have the metal atoms embraced by porphyrin rings and all have been made by J. P. Collman et al.546-548,551 Those that contain molybdenum are (OEP)MoRu(TPP), [(OEP)MoRu(TPP)]PF6, (OEP)MoOs(OEP), [(OEP)MoOs(TPP)]PF6, [(TPP)MoOs(OEP)]PF6 and [(TPP)MoRe(OEP)]PF6. Their electronic structures are probably all as expected; the physical properties do not suggest otherwise.
4.4.5 An overview of Mo–Mo bond lengths in Mo24+ compounds
At the end of 2001, a search of the Cambridge Crystallographic Database was made to determine the range and distribution of Mo–Mo distances in compounds with Mo24+ cores.552 This resulted in 465 compounds for which both the reported distances and the assignment of an Mo24+ core are believed to be correct. A histogram of these data is shown in Fig. 4.28. All of the distances have been rounded off to the second decimal place. In the range of 2.18-2.19 Å are nine compounds in which the torsion angles about the Mo–Mo bond are 26-40°, and thus a major part (40-70%) of the β bonding has been abolished. Were it not for this, these distances would probably have been 0.03-0.05 Å shorter. For nearly all of the remaining “long” bonds, there is some plausible reason for elongation.
Fig. 4.28. A histogram of Mo-Mo quadruple bond lengths.
The conclusion of this survey is that the “normal” range for Mo24+ bond lengths is 2.06 to 2.17 Å. Within this range the histogram shows a bimodal distribution, which can be ascribed to the fact that paddlewheel compounds tend to have shorter bonds (2.06-2.12 Å). These conclusions, while not to be taken as inviolable rules, provide a reliable guide to the distances that may reasonably be expected in compounds to be reported in the future.
4.5Higher-order Arrays of Dimolybdenum Units
4.5.1 General concepts
The terms supramolecular or higher-order array are used to designate any conglomeration of two or more M2n+ (usually with n = 4) units. We are concerned here with arrays of Mo2n+ units, but such arrays have also been made with other species of M2n+ units (W2n+, Re2n+, Rh24+, Ru25+) and are discussed in their appropriate chapters.
