Radiation Physics for Medical Physiscists - E.B. Podgorsak
.pdf8.18 General Aspects of Radioactive Decay |
349 |
The stable nuclides contain a balanced configuration of protons and neutrons and follow a curve of stability on the graph. The curve of stability follows Z ≈ N for low Z nuclides and then slowly transforms into N ≈ 1.5Z with increasing Z.
•Below the curve of stability are neutron-rich radionuclides. Most of neutron-rich radionuclides undergo a nuclear transmutation by β− decay but a few do so by direct neutron emission.
•Above the curve of stability are proton-rich radionuclides. Most of protonrich radionuclides undergo a nuclear transmutation by β+ decay or electron capture but a few do so by direct emission of one proton or even two protons.
•All nuclides with Z > 82 undergo α decay or spontaneous fission and some may also undergo β decay.
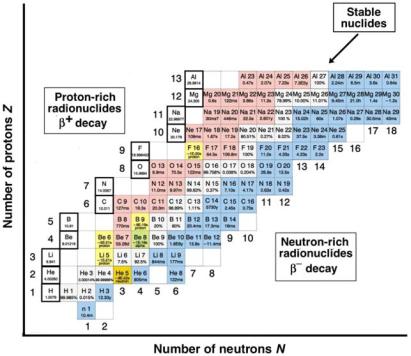
A small part of a simplified complete Segr` Chart is shown in Fig. 8.28 for 1 ≤ Z ≤ 13 and 0 ≤ N ≤ 18 (from hydrogen to aluminum). The stable nuclides are shown in light grey squares indicating the curve of stability that is given by Z ≈ N for low atomic number elements; neutron-rich and protonrich radionuclides are shown in dark grey squares below and above the region of stability, respectively. For stable nuclides their relative abundance is given; for radionuclides the half-life is given. The black framed squares above the region of the proton-rich radionuclides present the first 13 elements of the Periodic Table of Elements along with their nuclear mass in u given as the average for all stable isotopes of a given nuclear species.
As suggested by the individual rows in Fig. 8.28 (Z = const), a given atomic species in general consists of one or more stable isotopes and several radioactive isotopes; neutron-rich isotopes to the right of the stable ones and proton-rich to the left. For example, aluminum has only one stable isotope
(2713Al); hydrogen has two (11H and 21H) and oxygen has three (168 O, 178 O 1818O). On the other hand, hydrogen has one radioactive isotope, the neutron-rich
(31H), while aluminum has 4 neutron-rich isotopes and 4 proton-rich isotopes.
8.18 General Aspects of Radioactive Decay
Nuclear physics has come a long way since Ernest Rutherford’s momentous discovery that most of the atomic mass is concentrated in the atomic nucleus which has a size of the order of 1 fm = 10−15 m in comparison to the atomic
˚ −10
size of the order of 1 A = 10 m. The atomic nucleus consists of nucleons
– positively charged protons and neutral neutrons, and each nuclear species is characterized with a unique combination of the number of protons (atomic number) Z and number of neutrons N , the sum of which gives the number of nucleous A = Z + N (atomic mass number).
Nucleons are bound together into the nucleus by the strong nuclear force which, in comparison to the proton-proton Coulomb repulsive force, is at
350 8 Radioactivity
Fig. 8.28. A portion of the Chart of the Nuclides (Segr` Chart) for nuclides with proton numbers Z from 1 through 13, neutron numbers N from 0 through 18, and atomic mass numbers A from 1 through 31. Stable nuclides are shown with light pixel squares, radionuclides with darker pixel squares. Relative natural abundance is shown for the stable nuclides; half-lives and modes of decay are shown for radionuclides. The black-framed squares give the mean atomic mass in u for nuclides from hydrogen to aluminum. Each horizontal row (Z = const) represents one element including all stable and radioactive isotopes. Each vertical column (N = const) represents nuclides with the same neutron numbers (isotones)
least two orders of magnitude larger but of extremely short range of a few femtometers. To bind the nucleons into a stable nucleus a delicate equilibrium between the number of protons and the number of neutrons must exist. As evident from Figs. 8.27 and 8.28, for light (low A) nuclear species, a stable nucleus is formed from an equal number of protons and neutrons (Z = N ). Above the nucleon number A ≈ 40, more neutrons than protons must constitute the nucleus to form a stable configuration in order to overcome the Coulomb repulsion of the charged protons.
If the optimal equilibrium between protons and neutrons does not exist, the nucleus is unstable (radioactive) and decays with a specific decay constant into a more stable configuration that may also be unstable and decays further, forming a decay chain that eventually ends with a stable nuclide.
8.18 General Aspects of Radioactive Decay |
351 |
As discussed in detail in this chapter, nine main processes are available to unstable nuclei (radionuclides) to advance toward a more stable nuclear configuration; for a given radionuclide generally only one type or at most two types of decay process will occur.
Nuclides with an excess number of neutrons are referred to as neutronrich; nuclides with an excess of protons are referred to as proton-rich.
•For a slight imbalance, radionuclides will decay by beta decay characterized by transformation of a proton into a neutron in β+ decay and a transformation of a neutron into a proton in β− decay.
•For a large imbalance, the radionuclides will decay by emission of nucleons: α particles in alpha decay, protons in proton emission decay, and neutrons in neutron emission decay. For very large atomic mass number nuclides (A > 230) spontaneous fission which competes with α decay is also possible.
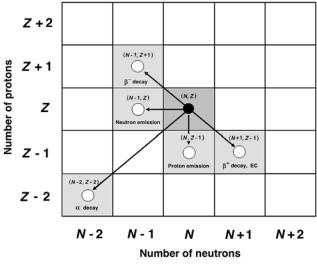
Figure 8.29 shows schematically the decay paths (except for the spontaneous fission) possibly open to a radionuclide (N, Z) in its transition toward a more stable configuration. The following general features of radioactive decay processes are noted:
1.When radionuclide (Z, N ) is below the curve of stability (i.e., is neutronrich), the β− decay and neutron emission are possible means to attain
a more stable configuration. The resulting nucleus will be characterized by (Z + 1, N − 1) for β− decay and by (Z, N − 1) for neutron emission decay.
2.When radionuclide (Z, N ) is above the curve of stability (i.e., is protonrich), the β+ decay, electron capture or proton emission may be possible means to attain a more stable configuration. The resulting nucleus will be characterized by (Z − 1, N + 1) for β+ decay and electron capture, and by (Z − 1, N ) for proton emission decay.
3.Proton and neutron emission decays are much less common than α and β decays and occur only in artificially produced radionuclides. The main characteristics of radionuclides which decay by proton or neutron emission are an extreme imbalance between the number of protons and the
number of neutrons in their nuclei as well as very short half-lives.
4. In addition to β decay the radionuclides (Z, N ) with Z < 83 may decay by α decay or spontaneous fission. In α decay the resulting nucleus is characterized by (Z − 2, N − 2), in contrast to spontaneous fission where the resulting nuclei are much lighter than the parent nucleus.
5.In gamma decay and internal conversion decay the parent nucleus is excited and undergoes a de-excitation process by emitting a γ photon or a conversion electron, respectively. Both the parent and the daughter nuclei are characterized by (Z, N ), since the number of protons as well as the number of neutrons does not change in the decay process.
352 8 Radioactivity
Fig. 8.29. Decay paths possibly available in the Chart of the Nuclides (Segr` Chart) to a parent radionuclide (N, Z) in its quest to attain a more stable configuration. The parent radionuclide is shown by the solid black circle, the daughter nuclides are shown by open circles
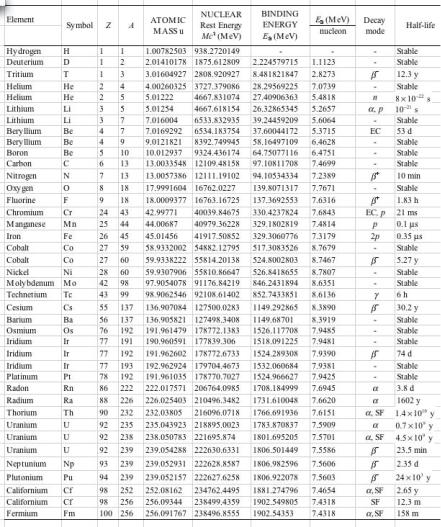
Table 8.6 lists the main attributes of nuclides presented in this chapter. The atomic masses M(u) were obtained from the tabulated data provided by the NIST. For a given nuclide, its nuclear rest energy M c2 is calculated from (8.219), its total binding energy EB from (8.220) and its binding energy per nucleon EB/A from (8.221). These data can be used to determine the decay energies for the radioactive decay examples presented in this chapter.
A summary of the main characteristics of the eight most common radioactive decay modes is given in Table 8.7 which for each mode provides expressions for the basic relationship, the decay energy as well as the energy of the decay products (daughter nucleus and emitted particles). In radioactive decay the daughter recoil kinetic energy (EK)D is generally ignored when determining the energy of the other, lighter decay products. However, we must keep in mind that in α decay as well as in proton and neutron emission decay (EK)D is of the order of 100 keV, while in other radioactive decay modes, except for the spontaneous fission, it is of the order of 10 eV to 100 eV. Thus the daughter recoil kinetic energy in α decay and in proton and neutron emission decay is not negligible and should be accounted for, while for the other common radioactive decays it may be ignored.
Table 8.8 presents a summary of the main attributes of the nine most common radioactive decay modes showing the changes in Z and N as well as the expressions for the decay energy Q calculated using either the nuclear masses M or the atomic masses M.
8.18 General Aspects of Radioactive Decay |
353 |
Table 8.6. Main attributes of nuclides presented in this chapter
Data for atomic masses were obtained from the NIST. M stands for the nuclear rest mass; M for the atomic rest mass. The nuclear mass-energy is calculated as follows:
M c2 = Mc2 − Zmec2 = (M × 931.494043 MeV/u) − (Z × 0.510999 MeV) |
(8.219) |
and the nuclear binding energies are calculated as: |
|
EB = Zmpc2 + (A − Z)mnc2 − M c2 |
(8.220) |
and |
|
EB/nucleon = EB/A . |
(8.221) |
The rest energies of the proton, neutron and electron are given as follows:
mPc2 = 938.2703 MeV, mnc2 = 939.56536 MeV, mec2 = 0.5109998918 MeV.