
- •VOLUME 3
- •CONTRIBUTOR LIST
- •PREFACE
- •LIST OF ARTICLES
- •ABBREVIATIONS AND ACRONYMS
- •CONVERSION FACTORS AND UNIT SYMBOLS
- •EDUCATION, COMPUTERS IN.
- •ELECTROANALGESIA, SYSTEMIC
- •ELECTROCARDIOGRAPHY, COMPUTERS IN
- •ELECTROCONVULSIVE THERAPHY
- •ELECTRODES.
- •ELECTROENCEPHALOGRAPHY
- •ELECTROGASTROGRAM
- •ELECTROMAGNETIC FLOWMETER.
- •ELECTROMYOGRAPHY
- •ELECTRON MICROSCOPY.
- •ELECTRONEUROGRAPHY
- •ELECTROPHORESIS
- •ELECTROPHYSIOLOGY
- •ELECTRORETINOGRAPHY
- •ELECTROSHOCK THERAPY.
- •ELECTROSTIMULATION OF SPINAL CORD.
- •ELECTROSURGICAL UNIT (ESU)
- •EMERGENCY MEDICAL CARE.
- •ENDOSCOPES
- •ENGINEERED TISSUE
- •ENVIRONMENTAL CONTROL
- •EQUIPMENT ACQUISITION
- •EQUIPMENT MAINTENANCE, BIOMEDICAL
- •ERGONOMICS.
- •ESOPHAGEAL MANOMETRY
- •EVENT-RELATED POTENTIALS.
- •EVOKED POTENTIALS
- •EXERCISE FITNESS, BIOMECHANICS OF.
- •EXERCISE, THERAPEUTIC.
- •EXERCISE STRESS TESTING
- •EYE MOVEMENT, MEASUREMENT TECHNIQUES FOR
- •FETAL MONITORING
- •FETAL SURGERY.
- •FEVER THERAPY.
- •FIBER OPTICS IN MEDICINE
- •FICK TECHNIQUE.
- •FITNESS TECHNOLOGY.
- •FIXATION OF ORTHOPEDIC PROSTHESES.
- •FLAME ATOMIC EMISSON SPECTROMETRY AND ATOMIC ABSORPTION SPECTROMETRY
- •FLAME PHOTOMETRY.
- •FLOWMETERS
- •FLOWMETERS, RESPIRATORY.
- •FLUORESCENCE MEASUREMENTS
- •FLUORESCENCE MICROSCOPY.
- •FLUORESCENCE SPECTROSCOPY.
- •FLUORIMETRY.
- •FRACTURE, ELECTRICAL TREATMENT OF.
- •FUNCTIONAL ELECTRICAL STIMULATION
- •GAMMA CAMERA.
- •GAMMA KNIFE
- •GAS AND VACUUM SYSTEMS, CENTRALLY PIPED MEDICAL
- •GAS EXCHANGE.
- •GASTROINTESTINAL HEMORRHAGE
- •GEL FILTRATION CHROMATOGRAPHY.
- •GLUCOSE SENSORS
- •HBO THERAPY.
- •HEARING IMPAIRMENT.
- •HEART RATE, FETAL, MONITORING OF.
- •HEART VALVE PROSTHESES
- •HEART VALVE PROSTHESES, IN VITRO FLOW DYNAMICS OF
- •HEART VALVES, PROSTHETIC
- •HEART VIBRATION.
- •HEART, ARTIFICIAL
- •HEART–LUNG MACHINES
- •HEAT AND COLD, THERAPEUTIC
- •HEAVY ION RADIOTHERAPY.
- •HEMODYNAMICS
- •HEMODYNAMIC MONITORING.
- •HIGH FREQUENCY VENTILATION
- •HIP JOINTS, ARTIFICIAL
- •HIP REPLACEMENT, TOTAL.
- •HOLTER MONITORING.
- •HOME HEALTH CARE DEVICES
- •HOSPITAL SAFETY PROGRAM.
- •HUMAN FACTORS IN MEDICAL DEVICES
- •HUMAN SPINE, BIOMECHANICS OF
384 GASTROINTESTINAL HEMORRHAGE
vacuum systems, as well as the high reliance now placed on these systems by medical–surgical–nursing staff. In the former, everyone is affected by the hazards; in the latter, failure of these systems can place patients at considerable medical risk.
Like any system, periodic maintenance is necessary in order to assure continuous, and optimum and safe level of operation. For piped gas or vacuum systems, this includes visual inspection of exposed pipes and outlets–inlets, sampling of gases (gas systems), measurement of pressures (gas systems), measurement of flow rates (vacuum systems), and testing of alarms. Guidance on this subject is included in such documents as NFPA 99, Standard for Health Care Facilities (11).
While today’s standards assure a high probability of a safe and reliable system, mechanical failures can and do occur, and human error or abuse still remain. Thus, should a fault of some kind occur, or a wrong connection be made, periodic maintenance should detect the condition so that corrective action can be taken before a serious incident occurs. This maintenance is particularly necessary whenever either system is breached for upgrading, component maintenance occurs, or system expansion purposesis made. The value of these systems in the treatment of patients demands no less.
Originial manuscript for this article was reviewed for technical accuracy by John M.R. Bruner, M.D., W.E. Doering, William H.L. Dornette, M.D., James F. Ferguson, Edwin P. Knox, (the late) David A. McWhinnie, Jr., Ralph Milliken, M.D., and (the late) Carl Walter, M.D.
BIBLIOGRAPHY
Cited References
1.Compressed Gas Association. Commodity Specification of Air. G-7.1, Arlington Chantilly (VA): CGA; 1973–2004.
2.Compressed Gas Association. Standard for the Installation of Nitrous Oxide System at Consumer Sites. G-8.1. Chantilly Arlington (VA): CGA; 1979–1990.
3.Compressed Gas Association. Standard Color-Marking of Compressed Gas Cylinders Intended for Medical Use in the OR, C-9. ChantillyArlington (VA): CGA; 1982–2004.
4.Compressed Gas Association. Commodity Specification for Nitrogen, G-10.1. ChantillyArlington (VA): CGA; 1985–2004.
5.Compressed Gas Association. Compressed Gas Cylinder Valve Outlet and Inlet Connections. V-1. ChantillyArlington (VA): CGA; 1977–2003.
6.Compressed Gas Association. Diameter Index Safety System, V-5. ChantillyArlington (VA): CGA; 1978–2005.
7.American Society of Sanitary Engineering. Professional Qualifications Standard for Medical Gas Systems, Installer, Inspectors, Verifiers, Maintenance Personnel and Instructors. ASSE, Series 6000: Westlake (OH); 2004.
8.American Society for Testing and Materials. Specification for Seamless Copper Water Tube. B-88, Philadelphia: ASTM; 1986–2003.
9.American Society for Testing and Materials. Specifications for Seamless Copper tube for Medical Gas Systems. B-819, Philadelphia: ASTM; 1992.
10.American Society for Testing and Materials. Standard Test Method for Behavior of Materials in a Vertical Tube Furnace at 7508C, E-136, Philadelphia: ASTM; 1982–2004.
11.National Fire Protection Association. Standard for Health Care Facilities (which includes criteria on piped medical gas systems, piped medical–surgical vacuum systems, and emergency electrical power), NFPA 99. Quincy (MA): NFPA; 1987–2005.
12.National Fire Protection Association. Standard for Bulk Oxygen System at Consumer Sites, NFPA 50. Quincy (MA): NFPA; 1985 (now included in NFPA 55, Standard for the Storage, Use, and Handling of Compressed Gases and Cryogenic Fluids in Portable and Stationary Containers, Cylinders, and Tanks; 2005).
13.National Fire Protection Association. National Electrical Code. NFPA 70, Quincy (MA): NFPA; 1987–2005.
14.National Fire Protection Association. Life Safety Code. NFPA 101, Quincy (MA): NFPA; 1985–2003.
15.National Fire Protection Association. Standard on Types of Building Construction. NFPA 220, Quincy (MA): NFPA; 1985–1999.
16.National Fire Protection Association. Standard Test Method for Potential Heat of Building Materials. NFPA 259, Quincy (MA): NFPA; 1987–2003.
Reading List
National Fire Protection Association. Historical Proceedings. Annual Meeting, Quincy (MA): NFPA; 1933.
National Fire Protection Association. Historical Proceedings. Annual Meeting, Quincy (MA): NFPA; 1934.
National Fire Protection Association. Historical Proceedings. Annual Meeting, Quincy (MA): NFPA; 1950.
National Fire Protection Association. Historical Proceedings. Annual Meeting, Quincy (MA): NFPA; 1951.
American Welding Society. Specification for Brazing Metal. A5.8, Miami (FL): AWS; 1981–2003.
American Society of Mechanical Engineers. Boiler and Pressure Vessel Code. New York: ASME; 1986–2001.
See also CODES AND REGULATIONS: MEDICAL DEVICES; EQUIPMENT MAINTENANCE, BIOMEDICAL; SAFETY PROGRAM, HOSPITAL.
GAS EXCHANGE. See RESPIRATORY MECHANICS AND GAS
EXCHANGE.
GASTROINTESTINAL HEMORRHAGE
R.C. BRITT
L.D. BRITT
Eastern Virginia Medical School
Norfolk, Virginia
INTRODUCTION
Gastrointestinal (GI) hemorrhage is a common medical problem, with significant morbidity and mortality. Traditionally, GI hemorrhage was managed by medically supporting the patient until the bleeding stopped or surgical intervention was undertaken. The modern day management of GI hemorrhage involves a multidisciplinary approach, including gastroenterologists, surgeons, interventional radiologists, primary care physicians, and intensivists. Despite the evolution in management of GI hemorrhage, the mortality rate has remained fairly constant, concentrated in the elderly with greater comorbidity (1). Additionally,
medical advances, e.g., proton pump inhibitors, H2 blockers, antimicrobial treatment of Helicobacter pylori, and endoscopic management have led to a decrease in the number of operations for hemorrhage, but not in the actual number of hemorrhages (2). The incidence of upper GI bleeding has remained relatively constant at 100–150/ 100,000 people (3), with an estimated 300,000–350,000 admissions annually and a mortality rate of 7–10% (4). Lower GI hemorrhage accounts for 20–30% of GI hemorrhage, and typically has a lower mortality rate than upper GI bleeding.
There are three major categories of GI hemorrhage, including esophageal variceal bleeding, nonvariceal upper GI bleeding, and lower GI bleeding. Typically, upper GI bleeding is classified as that bleeding occurring from a source proximal to the ligament of Treitz, with lower GI bleeding occurring distally. When bleeding occurs in the upper GI tract, it can be vomited as bright red blood, referred to as hematemesis. Slower bleeding from the upper GI tract is often referred to as ‘‘coffee-ground emesis’’, which refers to the vomiting of partially digested blood. Black, tarry stool is referred to as melena, and usually originates from an upper GI source, with the black color due to the action of acid on hemoglobin. Visible blood in the stool, or bright red blood per rectum, is referred to as hematochezia. Hematochezia is usually indicative of lower GI bleeding, although brisk upper GI bleeding may also present as hematochezia. The stool may also be maroon, suggesting the blood has mixed with liquid feces, usually in the right colon.
INITIAL EVALUATION AND RESUSCITATION
Upon presentation with GI hemorrhage, two large-bore (16 gauge or larger) peripheral IVs should be placed and intravascular volume resuscitation initiated with an isotonic solution. Lactated Ringers is frequently preferred to 0.9% Normal Saline because the sodium and chloride concentrations more closely approximate whole blood. The ABCs of resuscitation are a priority in the initial evaluation of the massive GI bleed, with careful attention given to the airway because of the high incidence of aspiration. The patient must be carefully monitored to ensure the adequacy of resuscitation. In the presence of continued rapid bleeding or failure of the vital signs to improve following 2 L of crystalloid solution, the patient should also begin receiving blood. If type-specific blood is not yet available, the patient may receive O negative blood.
On presentation, blood is drawn for hematocrit, platelets, coagulation profile, electrolytes, liver function tests, and a type and cross. Caution must be used when evaluating the initial hematocrit, as this does not accurately reflect the true blood volume with ongoing hemorrhage. A foley catheter should be inserted to monitor for adequate urine output as a marker for adequate resuscitation. An NG tube should be inserted to evaluate for the presence of upper GI bleeding, as bright red blood per NG tube indicates recent or active bleeding. While clear, bilious aspirate usually indicates that the source of bleeding is not upper GI, this is not a definite as absence of blood on
GASTROINTESTINAL HEMORRHAGE |
385 |
nasogastric aspirate is associated with a 16% rate of actively bleeding lesions found on upper endoscopy (5).
A thorough history is paramount when evaluating a patient presenting with GI hemorrhage. The clinical history may suggest the etiology of hemorrhage, as well as offer prognostic indicators. Important features in the history include a history of previous bleeding, history of peptic ulcer disease, history of cirrhosis or hepatitis, and a history of alcohol abuse. Also important is a history of medication use, particularly aspirin, nonsteroidals, and anticoagulants. Symptoms the patient experiences prior to the onset of bleeding, such as, the presence or absence of abdominal pain, can also be useful in the diagnosis.
A comprehensive physical exam must be done to evaluate the severity of the hemorrhage, as well as to assess for potential etiology. Massive hemorrhage is associated with signs and symptoms of shock, including tachycardia, narrow pulse pressure, hypotension, and cool, clammy extremities. The rectal exam may reveal the presence of bright red blood or melena, as well as evidence of bleeding hemorrhoids in a patient with bright red blood per rectum. Physical exam is also useful to evaluate for stigmata of liver failure and portal hypertension, such as, jaundice, ascites, telangiectasia, hepatosplenomegaly, dilated abdominal wall veins, and large hemorrhoidal veins.
When faced with a patient presenting with GI hemorrhage, the complete history and physical exam will help direct further management by assessing the likely source of bleed. The initial questions that must be answered to determine management priorities include whether the likely source of hemorrhage is from the upper or lower GI tract, and if the bleeding is from an upper source, whether the bleed is likely variceal or nonvariceal (Table 1).
UPPER GASTROINTESTINAL BLEEDING
Upper gastrointestinal bleeding is shown in Table 2.
Gastroesophageal Varices
Portal hypertension, defined as an increase in pressure > 5 mmHg (0.666 kPa) in the portal venous system (6), can lead to acute variceal hemorrhage. Cirrhosis, related to either chronic alcohol abuse or hepatitis, is the most common cause of portal hypertension, and leads to an increased outflow resistance, which results in the formation of a collateral portosystemic circulation. Collaterals form most commonly in the gastroesophageal junction and form submucosal variceal veins. Patients with isolated splenic vein thrombosis often form submucosal varices in the fundus of the stomach. Some 30–60% of all cirrhotic patients will have varices at the time of diagnosis, and 5–8% develop new varices each year (7). One-third of patients with varices will experience variceal hemorrhage, with mortality from the first variceal bleed as high as 50% (8). Rebleeding occurs frequently, especially in the first 6 weeks. Risk factors for early rebleeding, within the first 6 weeks, include renal failure, large varices, and severe initial bleeding with hemoglobin < 8 g dL 1 at admission (6). The risk of late rebleeding is related to the severity of liver

386 GASTROINTESTINAL HEMORRHAGE
Table 1. Localization of Gastrointestinal Hemorrhage
Diagnosis |
History |
Physical Examination |
|
|
|
Esophagus
Nasopharyngeal bleeding |
Epistaxis |
Esophagogastric varices |
Alcoholism, lived in area where schistosomiasis |
|
is endemic, history of blood transfusions |
|
or hepatitis B |
Blood in nares, blood dripping down pharynx, evidence for telangiectasias
Stigmata of chronic liver disease, (e.g., gynecomastia, testicular atrophy, parotid enlargement Cachexia, Kaposi’s sarcoma, oral candidiasis)
Esophagitis |
Dysphagia, odynophagia; immunosuppressed, |
|
|
(e.g., AIDS); diabetes mellitus, lymphoma, |
|
|
elderly |
|
Esophageal neoplasm |
Progressive dysphagia for solids |
Cachexia |
Mallory–Weiss tear |
Retching or vomiting prior to hematemesis |
Not specific |
|
Stomach |
|
Acute gastric ulcer |
Intensive care unit setting |
Comatose, multiple burns, on respirator |
Chronic gastric ulcer |
Peak between 55 and 65 years old |
Not specific |
Acute hemorrhagic gastritis |
History of aspirin use, intensive care unit setting |
Similar to acute gastric ulcer |
Gastric neoplasm |
Weight loss, early satiety; obstructive symptoms |
Cachexia, Virchow’s node; abdominal |
|
|
mass |
Gastric angiodysplasia |
Elderly |
Aortic stenosis murmur |
Gastric telangiectasia |
Epistaxis, family history of Osler-Weber-Rendu |
Telangiectasias on lips, buccal |
|
disease or history of renal failure |
mucosa, palate |
|
Duodenum |
|
Duodenal ulcer |
Epigastric pain |
Not specific |
Aortoenteric fistula |
History of abdominal aortic aneurysm repair |
Laparotomy scar |
|
Colon |
|
Colonic neoplasm |
Often occult; if located in rectosigmoid |
Mass on rectal examination |
|
then may have obstructive symptoms |
|
Cecal angiodysplasia |
Elderly, recurrent bleeding, low grade |
Aortic stenosis murmur |
Colonic diverticuloses |
Severe, single episode of bright red blood per rectum |
Not specific |
|
|
|
failure, ongoing alcohol abuse, variceal size, and renal failure (6).
Variceal bleeding classically presents as massive, painless upper GI hemorrhage in a patient with known cirrhosis. The management of acute variceal bleeding requires attention to adequate resuscitation as well as control of the active bleeding and minimization of complications related to the bleed (Fig. 1). Early endoscopy is imperative for the successful management of variceal bleeding. Frequently,
Table 2. Upper Gastrointestinal Bleeding
Differential Diagnosis of Upper GI Hemorrhage
Gastroesophageal varices
Mallory–Weiss tear
Esophagitis
Neoplasm esophagus, stomach, small bowel
Gastritis: stress, alcoholic, drug-induced
Angiodysplasia of stomach, small bowel
Peptic ulcer disease: stomach, duodenum
Dieulafoy ulcer
Aortoenteric fistula
Hemobilia
endoscopy is performed in conjunction with pharmacologic therapy. Endoscopy is essential to confirm the diagnosis of bleeding varices, as many patients with cirrhosis bleed from a source other than varices. Endoscopic sclerotherapy is effective in managing active variceal hemorrhage 70–90% of the time, and is superior to balloon tamponade or vasopressin (6). Intravariceal and paravariceal injections are equally efficacious. Sclerotherapy should be repeated at 1 week, and then at 1–3 week intervals until the varices are obliterated. Endoscopic variceal band ligation achieves hemostasis 90% of the time, and is felt to have a lower rebleeding and complication rate than sclerotherapy (9).
Pharmacologic therapy is used in conjunction with early endoscopy in massive variceal bleeding. Vasopressin, which works to cause splanchnic and systemic vasoconstriction and thus decrease portal venous flow, was traditionally used to control hemorrhage, but its use is limited by systemic side effects in 20–30% of patients (10). Vasopressin causes systemic vasoconstriction, which is particularly problematic in patients with coronary artery disease, in which vasoconstriction may induce myocardial infarction. Simultaneous administration of intravenous nitroglycerine will minimize the cardiac complications
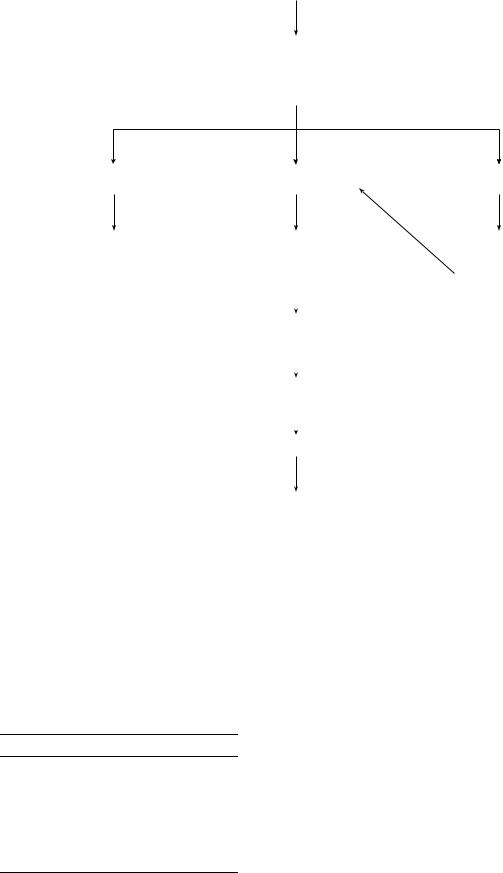
GASTROINTESTINAL HEMORRHAGE |
387 |
Acute Hemorrhage Gastroesophageal Varices
-Resuscitation
-Endoscopy with band ligation versus sclerotherapy
-Octreotide
No Further Bleeding |
Continued Hemorrhage |
Early rebleed |
Definitive Treatment |
- Balloon tamponade |
Endoscopy with |
|
- Endoscopic band |
- Octreotide |
band ligation or |
|
ligation |
|
|
sclerotherapy |
|
|
||
- Beta-blockers |
|
|
|
- Surgical Shunt |
|
|
|
|
|
|
|
- Child-Pugh class C |
Continued hemorrhage |
|
|
consider liver |
|
||
|
|
|
|
|
|
|
|
transplantation |
|
|
|
|
|
|
|
|
TIPS |
|
|
|
|
|
|
|
|
|
|
|
Continued hemorrhage |
|
|
Surgical Shunt
Figure 1. Management of acute variceal bleeding.
associated with vasopressin. Somatostatin, and its longacting analog Octreotide, work via inhibition of various vasodilatory hormones, and therefore inhibit splanchnic vasodilatation and decrease portal pressure. Somatostatin is as effective as vasopressin, but without the systemic side effects (11), and is currently the medication of choice to reduce portal pressure.
Occasionally, a patient presents with massive variceal hemorrhage not amenable to endoscopic or pharmacologic
Table 3. Lower Gastrointestinal Bleeding
Differential Diagnosis of Lower GI Hemorrhage
Colonic diverticular disease
Colonic arteriovenous malformations
Neoplasm: colon, small bowel
Meckel’s diverticulum
Ulcerative colitis
Crohn’s disease
Colitis: infectious, ischemic, radiation-induced
Internal hemorrhoidal disease
therapy. Balloon tamponade, generally done with the Sengstaken–Blakemore tube, can be used to achieve short-term hemostasis, which is successful 60–90% of the time (12). Caution must be taken to secure the airway with endotracheal intubation prior to placement of the tamponade balloon because of the high risk of aspiration. Care must be used to ensure that the gastric balloon is in the stomach prior to full insufflation, as migration or inflation of the gastric balloon in the esophagus can lead to esophageal rupture. The balloon can be left in place for 24 h, at which time endoscopic band ligation or sclerotherapy can be performed.
Bleeding that cannot be controlled by endoscopic therapy or that recurs should be managed with portal pressure reduction. The initial approach currently used is the transjugular intrahepatic portosystemic shunt (TIPS), which can be done with or without general anesthesia. Potential benefits to the use of general anesthesia include advanced management of fluid dynamics by the anesthesiologist and pain management for the patient. The TIPS method works by creating a channel between the hepatic and portal veins, which is kept patent by a metal stent, which achieves

388 GASTROINTESTINAL HEMORRHAGE
Acute Nonvariceal Upper GI Hemorrhage
-Resuscitation
-Endoscopy with injection therapy and thermal coagulation if active bleeding or visible vessel
-Proton Pump inhibitor therapy
rebleed
Repeat endoscopy with injection and thermal therapy
Continued bleeding or massive hemorrhage
Low risk surgical candidate |
High risk surgical patient |
||
|
|
|
|
|
|
|
|
Surgery |
Therapeutic angiography |
||
Figure 2. Management of acute nonvaricea bleeding.
hemostasis in 90% of patients (13). The downside of TIPS is related to shunt thrombosis, which can occur early or late and may result in recurrent variceal bleeding. Approximately 20% of patients by 1 year and 30% by 2 years experience recurrent bleeding related to shunt thrombosis (14,15). Complications related to TIPS procedures include a 30% rate of encephalopathy, procedure related complications including inadvertent puncture of the portal vein leading to massive hemorrhage, stent stenosis and malfunction, and TIPS-associated hemolysis.
Traditionally, reduction of portal pressure was achieved by surgical shunt procedures or devascularization. Surgical shunt procedures include nonselective shunts, which divert all the portal flow away from the liver, and selective shunts. Nonselective shunts include the portacaval end- to-side and side-to-side shunts, the central spleno-renal shunt, and the interposition portacaval shunt. Nonselective shunts are successful in achieving hemostasis in the actively bleeding patient, but frequently lead to hepatic encephalopathy as well as acceleration of liver failure. Selective shunts include the distal splenorenal shunt and the small-diameter mesocaval shunt. The selective shunts have a lower rate of encephalopathy, but are frequently complicated by uncontrollable ascites given the continued portal flow. Nonshunt operations, including esophageal transection and devascularization of the gastroesophageal junction are rarely used today. In the setting of emergent operation for ongoing hemorrhage, a nonselective portacaval shunt is most frequently employed. The distal splenorenal shunt is the most common shunt used for elective control.
Once control of active bleeding is achieved, the focus shifts to prevention of future bleeding. Endoscopic band
ligation is the treatment of choice for long-term management of variceal hemorrhage (16). Beta-blockers in combination with nitrates have been shown to synergistically lower portal pressures and thus decrease the risk of rebleeding. Surgical shunting is an option in patients refractory to endoscopic or pharmacologic therapy, with the distal splenorenal shunt the most frequently used for this indication. For patients with liver failure, liver transplantation is effective for both longterm prevention of bleeding as well as hepatic decompensation and death.
NONVARICEAL UPPER GI BLEEDS
Peptic Ulcer Disease
Peptic ulcer disease is the most common cause of upper GI hemorrhage; accounting for between 40 and 50% of all acute upper GI bleeds. Major complications related to peptic ulcer disease include perforation, obstruction, and hemorrhage and occur in 25% of patients, with hemorrhage the most common complication. Risk factors for peptic ulcer disease include infection with H. pylori, nonsteroidal antiinflammatory use, and physiologic stress related to critical illness. Medical advances including proton pump inhibitors and H2 blockers have led to a decreased need for operation for hemorrhage, but no decrease in the actual number of hemorrhages (17).
Hemorrhage related to peptic ulcer will classically present as hematemesis. In the setting of massive bleeding, the patient may also present with hematochezia. The patient may give a history of midepigastric abdominal pain preceding the bleeding. Important elements in the history include a history of peptic ulcer disease and recent usage of aspirin or nonsteroidal medications. Adverse clinical prognostic factors include age > 60 years, comorbid medical conditions, hemodynamic instability, hematemesis, or hematochezia, the need for emergency surgical interventions, and continued or recurrent bleeding (18).
The initial diagnostic test on all patients presenting with an upper GI bleed should be endoscopy. Endoscopy is the best test for determining the location and nature of the bleeding lesion, provides information regarding the risk of further bleeding, and allows for therapeutic interventions. Endoscopy should be performed urgently in all high-risk patients, and within 12–24 h for patients with acute, self-limited episodes of bleeding. The goal of endoscopy is to stop the active hemorrhage and reduce the risk of recurrent bleeding. Stigmata of recent hemorrhage (SRH) are endoscopically identified features that help determine which patients should receive endoscopic therapy. The SRH include active bleeding visible on endoscopy, visualization of a nonbleeding visible vessel, adherent clot, and a flat, pigmented spot (18). Certainly, patients with the major SRH including active bleeding or a visible vessel should undergo endoscopic therapy, as meta-analysis has shown a significant reduction in rates of continued or recurrent bleeding, emergency surgery, and mortality in those who received endoscopic therapy versus those who did not (19).
A variety of modalities exist for endoscopic therapy, including injection, thermal, and mechanical therapy. Epinephrine diluted 1:10,000 is the most frequently used

GASTROINTESTINAL HEMORRHAGE |
389 |
Acute Lower GI Hemorrhage
Resuscitation
Anorectal exam with anoscopy
No active bleeding |
Active bleeding |
Colonoscopy within 24 hours
Hemodynamics unstable
Angiography available |
No angiography |
||
|
|
||
|
|
available |
|
|
|
|
|
|
|
|
|
Angiographic |
Surgery (subtotal |
||
localization +/– therapy |
colectomy) |
||
|
|
|
|
|
|
|
|
Surgery if ongoing |
|
|
|
bleeding |
|
|
|
Hemodynamics stable
Bleeding scan
-Angiographic localization +/– therapy if bleeding scan +
-Colonoscopy to localize, +/– therapy
Surgery if ongoing hemorrhage or lesion found on colonoscopy requires resection
Figure 3. Management of acute lower gastrointestinal bleeding.
injection therapy, with injection into and adjacent to the bleeding point until hemostasis is achieved. Other agents used for injection therapy include ethanol, ethanolamine, thrombin, and fibrin. Thermal therapy is generally delivered by coaptive techniques, including bipolar electrocoagulation or heater probe. With coaptive coagulation, the probe is used to physically compress the vessel prior to delivery of heat to seal the vessel. Laser photocoagulation and argon beam plasma coagulation are noncoaptive techniques that are used less frequently. Mechanical therapy with hemoclips can also be used in bleeding, although the efficacy may be limited by ulcer location or a firm, scarred ulcer base preventing adequate application of the clips. A combination of injection therapy with epinephrine and bipolar thermal therapy is the most common endoscopic management of an acute bleed.
Despite initial success with endoscopic therapy, 15–20% of patients will rebleed, generally within the initial 72 h (18). While this was traditionally considered a surgical indication, endoscopic retreatment is now recommended for most patients. Repeat endoscopy rather than surgery was found in a prospective, randomized study to be associated with less complications and similar mortality (20). Surgical indications include massive hemorrhage
unresponsive to resuscitation and continued bleeding unresponsive to nonoperative management. For bleeding gastric ulcers, the operation of choice is a wedge resection to include the portion of the stomach containing the ulcer with or without vagotomy. For duodenal ulcers, truncal vagotomy, pyloroplasty, and direct oversewing of the bleeding ulcer via duodenotomy is the most common operation. Care must be taken to incorporate the proximal and distal gastroduodenal artery as well as the transverse pancreatic artery.
Therapeutic angiography is an option when therapeutic endoscopy is unsuccessful and may be performed prior to surgical intervention, as is effective, less invasive than surgery, and does not impact on the ability to surgically manage the bleeding if the intervention is unsuccessful. Angiographic options include selective intra-arterial vasopressin infusion or embolotherapy with microcoils, poly- (vinyl alcohol) (PVA) particles, or gelatin sponge. Embolization is considered the first line angiographic therapy, with success rates as high as 88% (21). Vasopressin is selectively infused after bleeding has been identified by contrast extravasation at an initial rate of 0.2 units per minute, with an increase to 0.4 units per minute then 0.6 units per minute if hemostasis is not achieved. The infusion
390 GASTROINTESTINAL HEMORRHAGE
is continued for 12–24 h, and then gradually tapered. Efficacy of vasopressin infusion ranges from 60 to 90% (22). Side effects related to selective infusion of vasopressin include abdominal cramping, fluid retention, hyponatremia, cardiac arrhythmias, and systemic hypertension. Vasopressin should not be used in patients with coronary artery disease because of the risk for myocardial ischemia.
Pharmacologic therapy to reduce gastric acidity is generally started as an adjunct to endoscopic therapy. The H2 blockers were found in meta-analysis to reduce the rate of continued bleeding, surgery, and death (23); however, a subsequent multicenter randomized trial found no difference in rebleeding rates in patients randomized to famotidine infusion versus placebo (24). Intravenous proton pump inhibitors have been shown to reduce rebleeding rates, length of hospital stay, and need for blood transfusion (25). Treatment with a proton pump inhibitor is generally started on admission for upper GI bleed, and continued as an adjunct to endoscopic therapy.
Mallory–Weiss Tear
The Mallory–Weiss syndrome describes acute upper GI bleeding that occurs after retching or vomiting, and was first described by Kenneth Mallory and Soma Weiss in 1929 (26). The increased intragastric pressure caused by vomiting causes mucosal lacerations, which are usually longitudinal. The typical presentation is a patient who initially vomits gastric material, followed by hematemesis and melena. Mallory–Weiss tears can also occur after anything that raises intragastric pressure, such as blunt abdominal trauma, severe coughing, childbirth, seizures, and closed chest cardiac compression. Mallory–Weiss tears classically occur at the gastroesophageal junction, but can occur in the distal esophagus. The lesion is common, occurring in 5–15% of patients presenting with upper GI bleeding. The majority of Mallory–Weiss tears will stop bleeding spontaneously, although some patients will require emergency treatment for ongoing hemorrhage. Endoscopic options for Mallory–Weiss bleeding include band ligation, epinephrine injection, and hemoclip application. In cases not amenable to endoscopic management, operative therapy involves oversewing the laceration via a gastrotomy.
Gastritis
Stress gastritis is associated with multiple superficial gastric ulcerations and is typically seen in the critically ill patient. Mechanical ventilation and coagulopathy increase the risk for hemorrhage in the critically ill. Prophylaxis with H2 blockers and proton pump inhibitors has led to a decrease in the incidence of stress gastritis in the critically ill. Bleeding from gastritis usually is self-limited, not requiring intervention. Early endoscopy is essential to establish the diagnosis and rule out other sources of upper GI bleeding. The patient should be started on pharmacologic therapy with either proton pump inhibitors or H2 blockers at a therapeutic dose. Endoscopic laser anticoagulation has been used for bleeding gastritis. Intraarterial infusion of vasopressin or selective embolization may also be used to arrest hemorrhage in gastritis. Ongoing hemorrhage not amenable to nonsurgical management is
operatively managed with vagotomy, pyloroplasty, and oversewing of the bleeding sites versus total gastrectomy. The mortality for a patient requiring surgical management of bleeding gastritis remains quite high.
Esophagitis
Esophagitis is an unusual cause of acute gastrointestinal bleeding, and only rarely occurs in association with severe reflux esophagitis. The history would be suggestive of gastroesophageal reflux, with symptoms, such as, heartburn, cough, and hoarseness. Rare causes of esophagitis associated with bleeding in the immunocompromised patient include infection with candida, herpes, or cytomegalovirus (27).
Neoplasm
Acute upper GI bleeding is a rare manifestation of esophageal neoplasms, with < 5% of esophageal malignancies presenting with an acute bleed. Occult, slow GI bleeding is much more common with esophageal neoplasms. Benign tumors of the esophagus include leiomyomas and polyps, and are very unlikely to present with GI bleeding. Esophageal hemangiomas, which constitute only 2–3% of benign esophageal tumors, may present with potentially massiveGIhemorrhage. Leiomyosarcomasare morelikelyto bleed than benign leiomyomas. When brisk bleeding occurs in the setting of esophageal cancer, one also must consider the erosion of the tumor into a major thoracic vessel.
Dieulafoy Vascular Malformation
Dieulafoy lesions are the result of arterioles of large diameter (1–3 mm) running through the submucosa, with erosion of the overlying mucosa resulting in bleeding. The mucosal defect is usually small, without evidence of chronic inflammation. Dieulafoy lesions generally present with brisk hemorrhage, due to their arterial nature. Diagnosis is made by upper endoscopy, with visualization of a small mucosal defect with brisk bleeding. Management is initially endoscopic with epinephrine injection and bipolar thermal therapy.Catheterembolizationisgenerallysuccessfulinpatients who fail endoscopic management. For patients requiring surgical management, the operation involves a wedge resection of the lesser curve of the stomach at the site of the lesion.
AORTOENTERIC FISTULA
Aortoenteric fistula classically occurs as a communication between a prosthetic aortic graft and the distal duodenum, and the diagnosis should be entertained in any patient presenting with an upper GI bleed who has undergone aortic reconstruction. The time period from aortic surgery to presentation is varied, and many patients present years down the road. The patient will frequently present initially with a sentinel bleed, which may be followed by massive upper GI hemorrhage. Upper endoscopy is paramount to making the diagnosis, as well as ruling out other sources of upper GI bleeding. Upon making the diagnosis of aortoenteric fistula, the optimal management is surgical, with removal of the aortic prosthesis, extra-anatomic bypass, and repair of the duodenum.
HEMOBILIA
Hemobilia classically presents as upper GI bleeding, melena, and biliary colic. Diagnosis is established by upper endoscopy, with visualization of blood from the ampulla. Endoscopic retrograde cholangiopancreatography can more clearly delineate the source of hemobilia. A variety of disease processes can lead to hemobilia, including hepatobiliary trauma, chronic cholelithiasis, pancreatic cancer, cholangiocarcinoma, and manipulation of the hepatobiliary tree. While hemobilia remains a rare cause of upper GI bleeding, the frequency is increasing related to increased manipulation of the hepatobiliary system and improved diagnostic modalities. Many cases of hemobilia will resolve without intervention. In the setting of ongoing hemorrhage, angiography with selective embolization of the bleeding vessel is the primary treatment modality. Surgery is reserved for failure of angiographic management.
LOWER GI BLEEDING
The passage of bright red or maroon blood via the rectum suggests a bleeding source distal to the ligament of Treitz, although blood can originate from any portion of the GI tract, depending on the rate of bleeding. Some 80–90% of lower GI bleeding will stop spontaneously. Initial resuscitation is similar to the patient presenting with upper GI bleeding, with hemodynamic assessment, establishment of appropriate access, and thorough history and physical exam. Visual inspection of the anorectal region, followed by anoscopy is essential to rule out a local anorectal condition such as hemorrhoids as the source of bleeding. A variety of modalities are available to further define the etiology of the bleeding, including endoscopy, nuclear medicine, angiography, and intraoperative localization.
The timing of colonoscopy for acute lower GI bleeding is controversial, with early (within 24 h of admission) colonoscopy increasingly advocated. Certainly, visualization may be difficult in a massively bleeding patient. Aggressive bowel prep can be given for 6–12 h prior to endoscopy, with the benefit of improved visualization. The benefit of early colonoscopy, similar to early upper endoscopy for upper GI bleed, is the opportunity for endoscopic diagnosis and therapy, using injection therapy and thermal modalities. Colonoscopy can directly visualize the bleeding source, which is beneficial in directing the surgeon in resection if the patient has continued or recurrent hemorrhage. Additionally, early colonoscopy may shorten hospital length of stay (28).
Nuclear scans can localize the site of lower GI bleeding and confirm active bleeding, with sensitivity to a rate of bleeding as low as 0.05–0.1 mL min 1. Bleeding scans use either 99mTc sulfur colloid or 99mTc-labeled erythrocytes, with radioactivity detected by a gamma camera, analyzed by computer, and recorded onto photographic film. The 99mTc sulfur colloid has the advantage of detection of bleeding as slow as 0.05 mL min 1, is inexpensive, and easy to prepare, but only will detect bleeding within 10 min of injection as it disappears quickly from the bloodstream (21). 99mTc-labeled erythrocytes detect bleeding as
GASTROINTESTINAL HEMORRHAGE |
391 |
slow as 0.1 mL min 1, and circulate within the bloodstream for 24 h. The 99mTc-labeled erythrocyte technique is gen-
erally considered the test of choice because of an increased sensitivity and specificity when compared with the 99mTc sulfur colloid (21). When a bleeding scan is positive, angiography or endoscopy is recommended to confirm the location of bleeding, to diagnose the specific cause, and to possible apply either endoscopic or angiographic therapy.
Angiography is advantageous because of the potential for both localization and treatment. Angiographic control can permit elective rather than emergent surgery in patients who are good surgical candidates, and can provide definitive treatment for poor surgical candidates. Bleeding can be detected at a rate as low as 0.5 mL min 1. The SMA is cannulated initially, with cannulation of the IMA if the SMA study is nondiagnostic. When bleeding is localized, either vasopressin infusion or superselective catheter embolization may be used. Vasopressin is used in a method similar to upper GI bleeding, with an infusion rate of 0.2– 0.4 units min 1. Efficacy varies from 47–92%, with rebleeding in up to 40% of patients (21). Vasopressin is particularly effective for bleeding from diverticula.
Angiographic embolization may be done with a variety of agents, including coil springs, gelatin sponge, cellulose, and (PVA). There is less collateral blood supply in the lower G tract than in the upper, so embolization was initially thought to be a salvage therapy for those patients who would not tolerate an operation. Recent innovations in catheter and guidewire design, however, have enabled the interventional radiologist to superselectively embolize the bleeding vasa recta, sparing the collateral vessels and thus minimizing ischemia. Several small studies have reported successful embolization without intestinal infarction (21), with combined results showing successful hemostasis in 34 of 37 patients. Superselective embolization with coaxial microcatheters is currently considered the optimal angiographic therapy.
Traditionally, emergency operations for lower GI bleeding were required in 10–25% of patients presenting with bleeding (29). Surgical indications traditionally include hemodynamic instability, ongoing transfusion requirements, and persistent or recurrence of hemorrhage. If the bleeding has not been localized, a total abdominal colectomy with ileorectal anastomosis or end ileostomy is performed. If the lesion has been localized to either the right or left side of the colon, a hemicolectomy may be performed. With the advances in angiography available, the surgical indications are evolving. If an angiographer is readily available, angiographic localization and therapy is a viable option even for the hemodynamically unstable or persistently bleeding patient, thus avoiding the high morbidity and mortality associated with emergent total colectomy in this patient population.
Diverticulosis
The most common cause of lower GI bleeding is diverticular disease. Diverticular disease increases with age and is present in 50% of people > 80 years. Less than 5% of these patients, however, will hemorrhage. While most diverticula are found distal to the splenic flexure, bleeding
392 GASTROINTESTINAL HEMORRHAGE
diverticula more frequently occur proximal to the splenic flexure. Classically, the patient will present with sudden onset of mild lower abdominal pain and the passage of maroon or bright red bloody stool per rectum. The majority of diverticular bleeds will stop spontaneously, with a recent study showing spontaneous resolution in 76% of patients (1). About 20–30% of patients will have a recurrent bleeding episode, of which the majority will again stop without intervention. Patients that have persistent or recurrent bleeding should be considered for surgical therapy, particularly if the site of bleeding has been localized. High risk surgical patients can be treated with angiographic or endoscopic therapy.
Angiodysplasia
Angiodysplasia arises from age-related degeneration of submucosal veins and overlying mucosal capillaries, with frequency increasing with age. The bleeding tends to be less severe than with diverticular bleeds, and frequently resolves spontaneously, although recurrence is common. Diagnosis can be made by colonoscopy, with electrocoagulation as definitive therapy. Angiography may also be used for diagnosis, with the angiographic hallmarks a vascular tuft from an irregular vessel, an early and intensely filling vein resulting from arteriovenous communication, and persistent venous filling (30). Angiographic therapy with vasopressin can be used for treatment.
Neoplasm
While polyps and cancers frequently present with blood per rectum, they rarely cause massive hemorrhage as the presenting symptom. Diagnosis is made with colonoscopy. Management of a polyp is via colonoscopic polypectomy, while cancer requires surgical resection. Occasionally, a patient will present up to 1 month following a polypectomy with lower GI bleeding, which should prompt colonoscopy and thermal or injection therapy to the bleeding polypectomy site.
Meckel’s Diverticulum
Meckel’s diverticulum is an unusual cause of GI bleeding, and usually occurs in the first decade of life. The etiology of the bleeding is ectopic gastric mucosa in the diverticulum with resultant ulceration of adjacent bowel. Diagnosis is usually demonstrated by nuclear scanning demonstrating the ectopic gastric mucosa. Management is with surgical resection of the diverticulum as well as the adjacent bowel.
Ischemic Colitis
Ischemic colitis generally presents with bloody diarrhea, and massive lower GI bleeding is rare in this population. The bloody diarrhea is due to mucosal sloughing. Ischemic colitis should be suspected in patients with a history of vascular disease and in critically ill, hypotensive patients with a low flow state. Diagnosis is made by flexible endoscopy showing evidence of ischemia. Management for early ischemia is resuscitation and improvement of blood flow. Advanced ischemia requires surgical resection of the necrotic portion of the bowel.
Inflammatory Bowel Disease
GI bleeding characterizes both Crohn’s disease and ulcerative colitis; however, massive bleeding is quite uncommon. The bleeding from inflammatory bowel disease is usually self-limited, and rarely acutely requires surgical attention. Diagnosis is made by colonoscopy, with identification of features unique to these entities and biopsy for pathology. Occasionally, ulcerative colitis will present fulminantly with massive hemorrhage and require surgical resection, consisting of total colectomy, end ileostomy, and Hartman’s pouch, leaving the possibility for future conversion to an ileo-pouch anal anastomosis. Both entities are managed with immunosuppressive medications.
BIBLIOGRAPHY
Cited References
1.Hamoui N, Docherty DO, Crookes PH. Gastrointestinal hemorrhage: is the surgeon obsolete? Emerg Med Clin N Am 2003;21:1017–1056.
2.Bardhan KD, et al. Changing patterns of admission and operations for duodenal ulcer. Br J Surg 1989;76:230–236.
3.Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population based study. Am J Gastroenterol 1995;90:206–210.
4.Yavorski R, et al. Analysis of 3294 cases of upper gastrointestinal bleeding in military medical facilities. Am J Gastroenterol 1995;90:568–573.
5.Gilbert DA, et al. The national ASGE survey on upper gastrointestinal bleeding III. Endoscopy in upper gastrointestinal bleeding. Gastrointest Endosc 1982;27:94
6.Comar KM, Sanyal AJ. Portal hypertensive bleeding. Gastroenterol Clin N Am 2003;32:1079–1105.
7.Lebrec D, et al. Portal hypertension, size of esophageal varices, and risk of gastrointestinal bleeding in alcoholic cirrhosis. Gastroenterology 1980;79:1139–1144.
8.Pagliaro L, et al. Prevention of the first bleed in cirrhosis. A metaanalysis of randomized trials of non-surgical treatment. Ann Intern Med 1992;117:59–70.
9.Stiegmann GV, Goff GS, Sun JH, Wilborn S. Endoscopic elastic band ligation for active variceal hemorrhage. Am Surg 1989;55:124–128.
10.Conn HO. Vasopressin and nitroglycerine in the treatment of bleeding varices: the bottom line. Hepatology 1986;6:523– 525.
11.Inperiale TF, Teran JC, McCullough AJ. A meta-analysis of somatostatin versus vasopressin in the management of acute esophageal variceal hemorrhage. Gastroenterology 1995;109: 1289–1294.
12.Feneyrou B, Hanana J, Daures JP, Prioton JB. Initial control of bleeding from esophageal varices with the SengstakenBlakemore tube: experience in 82 patients. Am J Surg 1988; 155:509–511.
13.LaBerge JM, et al. Creation of transjugular intrahepatic portosystemic shunts with the wallstent endoprothesis: results in 100 patients. Radiology 1993;187:413–420.
14.Sanyal AJ, et al. Transjugular intrahepatic portosystemic shunts compared with endoscopic sclerotherapy for the prevention of recurrent variceal hemorrhage: a randomized, controlled trial. Ann Intern Med 1997;126:849–857.
15.LaBerge JM, et al. Two-year outcome following transjugular intrahepatic portosystemic shunt for variceal bleeding: results in 90 patients. Gastroenterology 1995;108:1143–1151.
