
Principles and Applications of Asymmetric Synthesis
.pdf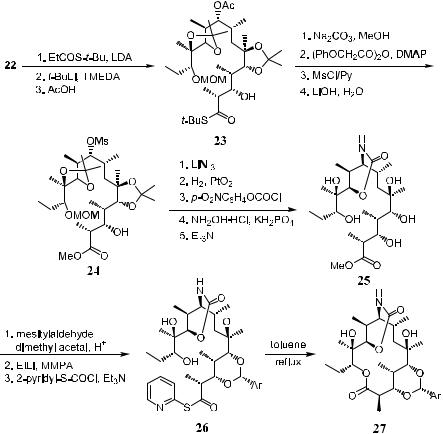
402 APPLICATIONS OF ASYMMETRIC REACTIONS
Scheme 7±6. Woodward's synthesis of erythromycin A.
can be used to fully control the stereochemistry. Splitting 29 results in fragments A (30) and B (31), which can be prepared from aldol reactions I (30) and II followed by III (31), respectively. Finally, the fragments are assembled via aldol reaction IV to give thio-seco acid 29.
Using an appropriate enolate reagent for each reaction, the two major fragments A (30) and B (31) can be constructed through one or two steps of aldol reactions (Scheme 7±8). It should be mentioned that compound 35, which is thoroughly discussed in Chapter 3, plays an important role in this synthesis.
Condensation of the two fragments A and B via aldol reaction, followed by macro-lactonization, completes the synthesis as shown in Scheme 7±9.
Note that in aldol reaction IV (from 31 to 42 in Scheme 7±9), the methodology di¨ers from that used in I, II, and III (see Scheme 7±7). Aldol reaction IV is also a double asymmetric reaction involving the coupling of two structurally

7.3 THE SYNTHESIS OF RIFAMYCIN S |
403 |
Scheme 7±7. Retro synthetic analysis of 6-deoxyerythronolide B.
pre®xed components. The coordination of Li‡ with the b-ethereal oxygen atom in aldehyde 31 is mainly responsible for the 17:1 stereoselectivity.
Thus, eight chiral centers have been created in molecule 28 with remarkable e½ciency and stereoselectivity by following the above steps.
7.3THE SYNTHESIS OF RIFAMYCIN S
Among the syntheses of complicated natural products, the total synthesis of rifamycin S (44) is another example that shows how a complicated structure can be constructed by applying the concept of double asymmetric synthesis (see Section 1.5.3 for double asymmetric synthesis). Rifamycin S is one of the ansamycin antibiotics, characterized by a distinct structural feature: a macro-

404 APPLICATIONS OF ASYMMETRIC REACTIONS
Scheme 7±8. Synthesis of fragment A and fragment B.
lactam with a long aliphatic ansachain (45) joined to an aromatic nucleus 46 at two nonadjacent positions. The intermediate 45 and its precursor 49 constitute another example of forming a subunit of the ®nal product via typical aldol reaction (Scheme 7±10).
7.3.1Kishi's Synthesis in 1980
The total synthesis of rifamycin S was one of Kishi's many achievements in organic synthesis.6 Kishi recognized that a certain type of (Z)-ole®n such as 51 tends to take a conformation in which C-1, C-2, C-3, and H-3 are nearly co-

7.3 THE SYNTHESIS OF RIFAMYCIN S |
405 |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 7±9. Synthesis of a derivative of the seco acid and ring closure to 6- deoxyerythronolide B.
planar due to alkylic strains. This conformation is likely to be retained in the transition states of many reactions, and in the process the two faces of the plane are di¨erentiated. Thus, hydroboration in Eq. 1 and epoxidation in Eq. 2 (Scheme 7±11) can be carried out with moderate diastereoselectivity.
When Kishi tried to apply these insights to rifamycin synthesis (Scheme 7±12), he at ®rst met the di½culty that direct epoxidation of the allylic alcohol 53, which was generated from 52 through a Wittig reaction, failed to introduce an a-epoxy group anti to the preexisting chiral methyl group in 52 due to interference of the OBn coordination with the oxidant. Thus, an indirect path had to be taken to prepare the rifamycin ansa chain. For his next attempts, as Scheme 7±12 indicates, a trimethylsilyl substituted allylic alcohol 55 was employed in the epoxidation reaction with m-CPBA, giving 56 with the introduced epoxy moiety at the a-position. Treatment of 56 with Fÿ to remove the silyl group was followed by epoxy ring opening with copper reagent, and this generated the aldol product 57.
In the application of the above discovery, …G†-3-benzyloxy-2-methyl propionaldehyde 52 is used as the starting material in the synthesis of rifamycin A. As outlined in Scheme 7±12, compound 52 is converted to allyic alcohol 55 via a series of chemical reactions. Epoxidation of 55 proceeded stereoselectively, giving a single epoxide that a¨ords 57 after subsequent treatment. Compound 57 may be converted to 58 upon acetonide formation.


|
|
|
|
|
|
|
|
|
|
|
7.3 THE SYNTHESIS OF RIFAMYCIN S |
407 |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 7±12
Scheme 7±13
The same strategy can be applied to complete the conversion from 58 to 61, as described in Scheme 7±13. Nearly the same sequence is performed for the conversion from 58 to 59, from 59 to 60, and from 60 to 61. Diethylzinc addition and subsequent methylation of the hydroxyl group provides 62 in a ratio of 4.6:1. In this manner, the synthesis of this key intermediate containing all eight chiral centers present in the ansa chain has been completed.

408 APPLICATIONS OF ASYMMETRIC REACTIONS
Scheme 7±14. Reagents and conditions: a (1) HCl; (2) t-BuCOCl, pyridine; (3) OsO4, KIO4; (4) MeSH, BF3 Et2O; (5) Me2C(OMe)2, CSA; (6) LiAlH4 (56% yield). b: (1) PDC; (2) Ph3PbCHCO2Et; (3) DIBAL±H. c: (1) PDC; (2) (MeO)2P(O)CH(Me)CN, t-BuOK; (3) DIBAL±H; (4) NaCN, MnO2, MeOH [45% yield from (G)-63]. d: (1) HgCl2, CaCO3; (2) NaBH4; (3) t-BuPh2SiCl; (4) Ac2O, pyridine; (5) Bu4NF; (6) methanesulfonyl chloride, Et3N; (7) MeSNa (69% yield).
Further transformation of 62 to the ansa chain …G†-66 is brie¯y summarized in Scheme 7±14 without a detailed discussion. Compound …G†-66 has now been properly functionalized for coupling with the aromatic unit of rifamycin.
7.3.2Kishi's Synthesis in 1981
One year after reporting the experiments outlined in Section 7.3.1, Kishi's group carried out an enantioselective version of the synthesis based on the experience obtained in the above synthesis of the racemate …G†-66.7 This version adopted the concept of double asymmetric induction demonstrated in the synthesis of 6-deoxythronolide B (see Section 7.2).
Scheme 7±15 shows the signi®cant improvement in overall stereoselectivity, due mainly to the adoption of the newly developed Sharpless asymmetric epoxidation. Compounds a-epoxy-67 and b-epoxy-67 can be readily obtained from 53 via the Sharpless reaction. Isomers of compounds 57 are then constructed via regioselective ring opening with a copper reagent.
Scheme 7±16 shows that a similar synthetic route leads to the asymmetric synthesis of optically active 62. The synthesis that began from homochiral aldehyde …ÿ†-52 used this newly discovered asymmetric epoxidation three times, 52 ! 58, 58 ! 68, and 68 ! 61, ®nishing the conversion from 52 to 61 by following a shortened route. The last chiral center to be built is C-27, and the addition of allyltin to the aldehyde derived from 61 proceeds with high stereoselectivity to give the chiral aliphatic segment 62.
The high stereoselectivity in the formation of 62 described in Scheme 7±16 can be explained by the trans-decalin type transition state 69, which ensures the desired con®guration at C-27.

7.3 THE SYNTHESIS OF RIFAMYCIN S |
409 |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 7±15
Scheme 7±16. Reagents and conditions: a: (1) (i-PrO)2P(O)CH2CO2Et, t-BuOK; (2) DIBAL±H; (3) …‡†-DET, Ti(OPri)4, TBHP; (4) LiCuMe2; (5) Me2CO, CSA; (6) Li, NH3(1) (57% yield). b: (1) DMSO, (COCl)2, Et3N, steps 2±5) as steps 1±4) in a; (6) t-BuPh2SiCl; (7) AcOH; (8) t-BuCOCl, pyridine; (9) Me2C(OMe)2, CSA; (10) LiAlH4 (63% yield). c: Steps 1±5 as in b but with …ÿ†-DET; (6) Me2C(OMe)2, CSA; (7) Bu4NF (68% yield). d: (1) DMSO, (COCl)2, Et3N; (2) CH2 bCHCH2I, SnCl2; (3) MeI, KH (65% yield).
7.3.3Masamune's Synthesis
The aldol reaction that establishes two chiral centers in one step has been applied to the synthesis of the ansa chain 66 by Kishi's group as discussed above. Seven chiral centers out of the eight present in the corresponding 66a can be constructed in a di¨erent way through a convergent series of four

410 APPLICATIONS OF ASYMMETRIC REACTIONS
Scheme 7±17. Retro synthetic analysis of 66a.
asymmetric cross aldol reactions. The intermediates can be prepared from a chiral boron reagent. The remaining chiral center C-23 can then be created by stereoselective reduction of the corresponding ketone 70. Finally, the introduction of a (Z,E )-dienolate moiety (C-15 to C-19) gives product 66a. The retro synthetic analysis is shown in Scheme 7±17.
Scheme 7±18 shows Masamune's implementation of this approach, beginning with aldehyde 71.8 This is reacted with boron enolate reagent (S)-35c (mentioned in Chapter 3; see Scheme 7±8 for its structure) and provides aldol product 72 with excellent enantioselectivity (100:1). Aldehyde 73 is obtained

|
|
|
|
7.3 THE SYNTHESIS OF RIFAMYCIN S |
411 |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 7±18. Synthesis of precursor 77. Reagents and conditions: a: (1) HF, CH3CN;
(2) NaIO4; (3) CH2N2, HBF4; (4) LiAlH4; (5) PCC (75% yield). b: (1) Et2CO, (Me2PhSi)2NLi; (2) TBSOTf (90% yield). c: (1) DIBAL±H; (2) Me2C(OMe)2, H2SO4;
(3) n-Bu4NF; (4) Me3SiCl, aqueous workup (85% yield). X ˆ TBS; SEM ˆ CH2OCH2CH2SiMe3.
from 72 through cleavage of the chiral auxiliary, oxidation, and subsequent protection of the hydroxyl group. Compound 75 is then produced via an asymmetric aldol reaction between aldehyde 73 and the (Z)-enolate of 3-pentanone and subsequent silylation. Coupling of the (Z)-enolate of 75 with 74 through a zirconium-mediated aldol reaction furnishes ketone 76, which can e½ciently be converted to 77 via reduction of the carbonyl group and subsequent protection and deprotection of its hydroxyl groups (Scheme 7±18).
Based on the method used in Kishi's synthesis, a diene moiety can be connected to 77 through routine synthetic chemistry, and this ®nishes the C-15 to C-19 subunit of intermediate 66. Conversion of 77 to 66 is brie¯y depicted in Scheme 7±19.
The interested reader is referred elsewhere for discussions of the synthesis of rifamycin.9
At this juncture, it is useful to look at Table 7±1, in which the syntheses of erythronolide and the ansa chain are used as examples to show that reagentcontrolled syntheses are clearly more advantageous than substrate-controlled reactions in terms of three criteria: the overall yield, overall stereoselectivity, and number of steps involved in each of the syntheses. A careful examination of Table 7±1 clearly shows the advantages of this strategy.
