
Principles and Applications of Asymmetric Synthesis
.pdf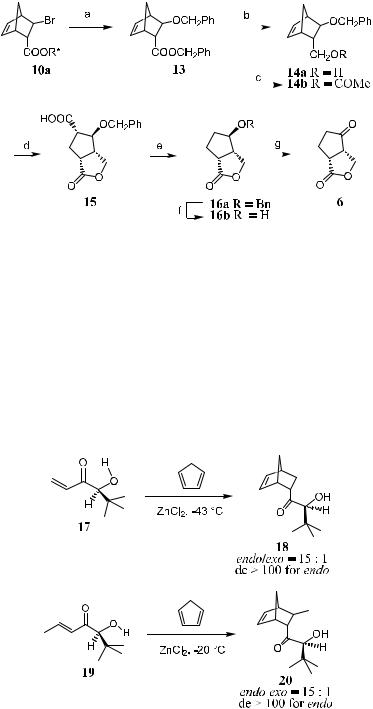
5.1 CHIRAL DIENOPHILES |
271 |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 5±5. Reagents and conditions: a: NaOCH2Ph, THF/DMF 5:1, ÿ10 C to room temperature. b: LiAlH4, Et2O, re¯ux. c: Ac2O, Py. d: (1) Cat. RuCl3/NaIO4, CCl4/ MeCN/H2O 1:1:1.5, room temperature. (2) LiOH, THF/H2O 5:4, 0 C. (3) 2N HCl, Et2O, 0 C to room temperature. e: (1) N-methylmorpholine, isobutylchloroformate, N- hydroxy-2-thiopyridone, THF, NEt3, THF, ÿ15 C, (2) t-butyl mercaptan, irradiation,
Ê
THF, room temperature. f: H2, 10% Pd/C, EtOH. g: Cat. (n-Pr)4NRuO4, NMO, 4 A MS, CH2Cl2, room temperature.
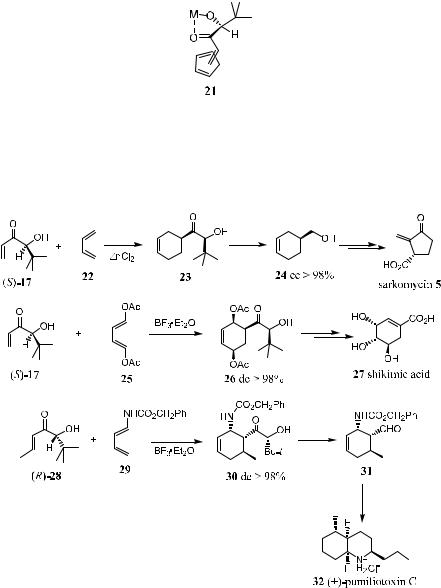
to the reacting site. Thus, compound 17 reacts with cyclopentadiene in the presence of ZnCl2 at ÿ43 C, a¨ording exclusively endo-adduct 18. The reaction proceeds quickly and completes within an hour. Similarly, compound 19, a homologue of 17, undergoes a Diels-Alder reaction giving compound 20 with similarly high stereoselectivity (Scheme 5±6).7
Scheme 5±6
272 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
The high enantioselectivity shown in the above reactions can be attributed to two important factors. First, coordination of the Lewis acid with the a-hydroxy ketone moiety of dienophile 17 or 19 leads to the formation of a rigid ®vemembered chelate 21. This chelate causes the di¨erentiation of the two diastereotopic faces of the enone system. Second, arising from the established absolute con®guration of 17 and 19, within 21, the Diels-Alder reaction proceeds with the enone fragment at its cisoid position (syn-planar).
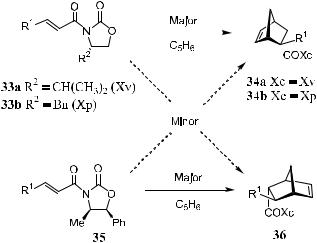
A variety of dienes have been subjected to Diels-Alder reactions with (S)-17 and (R)-28, giving almost pure single adducts in each case. Oxidative removal of the chiral auxiliary from the adduct provides the desired optically pure building blocks 23, 26, and 30. Subsequent conversions complete the synthesis of the desired natural products 5, 27, and 32 (Scheme 5±7).7
Scheme 5±7
5.1 CHIRAL DIENOPHILES |
273 |
||
|
|
|
|
|
|
|
|
Scheme 5±8
5.1.3Chiral a,b-Unsubstituted N-Acyloxazolidinones
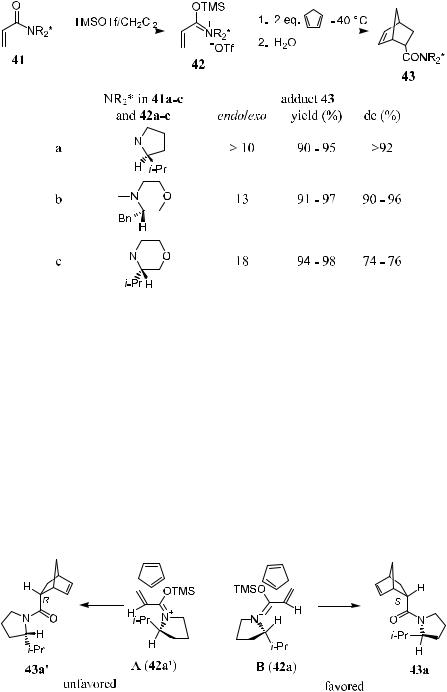
Chiral a,b-unsaturated N-acyloxazolidinones have been regarded as a complement for type I dienophile reagents. Evans et al.4 reported a Diels-Alder reaction promoted by dialkyl aluminum chloride. In this reaction, chiral a,b- unsaturated N-acyloxazolidinones were used as highly reactive and diastereoselective dienophiles. The stereogenic outcome of the Diels-Alder adducts 34 and 36 depends on the chirality at C-4 of the oxazolidinone auxiliaries. As shown in Scheme 5±8, all the diastereomeric adducts of 34a, 34b, and 36 can be obtained from the dienophiles 33a, 33b, or 35. The high stereoselectivity is a consequence of chloride ionization from 1:1 complex 38 (taking 33a as an example, where R1 ˆ CH3). As illustrated by the model in Figure 5±3, the resulting ionic dienophile 38 favors the Ca ±Si-face of the cyclopentadiene molecule leading to 34a as the major product. In accordance with this model, cycloaddition should occur selectively from the Ca ±Re-face of the crotonyl imide 35 to a¨ord the enantiomer of 34a (Xc ˆ OH) via adduct 36. The disadvantage of this Diels-Alder reaction is that it is limited to monosubstituted and (E )- disubstituted imide dienophiles.
Chiral oxazolidine compounds have also been used as chiral auxiliaries for asymmetric Diels-Alder reactions. Adam et al.8 demonstrated the cycloaddition of optically active 2,3-dimethyloxazolidine derivatives with singlet oxygen. As shown in Scheme 5±9, the reaction of chiral substrate 39 with singlet oxygen provides product 40 in high diastereomeric ratio.
5.1.4Chiral Alkoxy Iminium Salt
As depicted in Scheme 5±10, iminium salts such as 42 have been found to exhibit substantially increased reactivity in Diels-Alder reactions.9 This means

274 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Figure 5±3. Diastereoface selection in the cycloaddition process. Reprinted with permission by Am. Chem. Soc., Ref. 4.
Scheme 5±9. Reprinted with permission by Am. Chem. Soc., Ref. 8.
that a chiral amide such as 41 might be used for asymmetric Diels-Alder reactions. The asymmetric Diels-Alder reaction of 41a±c in the presence of TMStri¯ate yields amide compounds 43a±c in high yield and with the endocompound as the major product.
The high stereoselectivity of the products obtained can be explained by postulating transition states A and B (Fig. 5±4). In transition state A, the diene approaches in an endo fashion, from the more sterically hindered side of the dienophile; while in transition state B, although the diene again approaches in an endo fashion, it approaches from a less hindered side. Thus, transition state B has a lower energy and leads to product 43a with (S)-con®guration a to the
|
|
|
|
|
|
|
|
|
|
|
|
5.1 CHIRAL DIENOPHILES |
275 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 5±10
carbonyl group. The same rationale is applicable to 41b and 41c, which result in Diels-Alder products 43b and 43c with predominantly (R)-con®guration (Scheme 5±10). Because the morpholine and pyrrolidine chiral auxiliaries can readily be prepared in enantiomerically pure form, this method provides a route to both the (R)- and (S)-acids, which can be obtained from compound 43 upon removal of the chiral auxiliary.
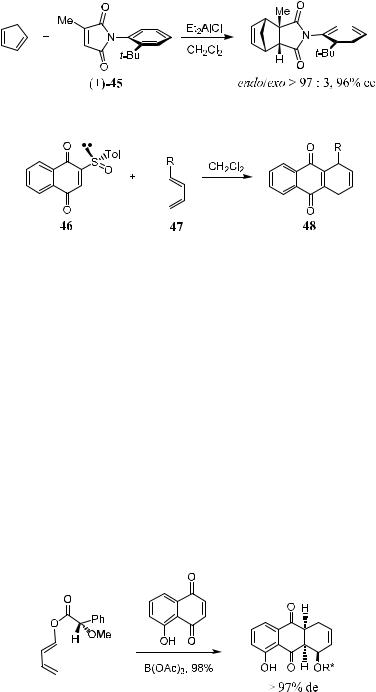
Recently, two new axially chiral compounds 44 and 45 have been prepared [44, N-acryl-N-allyl-o-t-butylanilide; 45, N-(o-t-butylphenyl)-2-methyl- maleimide]. This represents the ®rst instance of using nonbiaryl axially chiral ligands in asymmetric Dield-Alder reactions. In the presence of iodine, high endo-facial and diastereofacial selectivities have been obtained in 44/45-medi- ated reactions.10
Figure 5±4. The proposed transition states of 42a and 42a0 with cyclopentadiene.

276 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Asymmetric Diels-Alder reactions have been carried out to investigate the e½cacy of anilide 44 and imide 45 as dienophiles. In the presence of iodine, the asymmetric Diels-Alder reaction of anilide …‡†-44 shows a remarkable improvement in both reactivity and stereoselectivity (Table 5±1). Therefore, it is believed that, in the presence of I2, Diels-Alder reactions of N-allylic enamides take place through an activating process involving the formation of a cationic iodocyclization intermediate.
The Diels-Alder reaction of maleimide …‡†-45 with cyclopentadiene requires a prolonged reaction time (3 days) at room temperature in the absence of activating agents. The yield is around 80%. However, in the presence of Et2AlCl, the reaction can be completed with a quantitative yield within 20 minutes at room temperature (Scheme 5±11).
TABLE 5±1. Iodine-Mediated Asymmetric Diels-Alder Reaction of (B)-44
Entry |
Diene |
Major Product |
Yield (%) |
endo/exo |
ds (%) |
1 |
|
|
92 |
30 |
29/1 |
2 |
84 |
>50 |
14/1 |
3
87 |
20 |
Conditions: 44(1 mmol), I2 and diene (2 mmol), AcOEt (5 ml), ÿ78 C to room temperature. ds ˆ diastereoselectivity.
Reprinted with permission by Am. Chem. Soc., Ref. 10(b).
5.2 CHIRAL DIENES |
277 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 5±11
Scheme 5±12
5.1.5Chiral Sul®nyl-Substituted Compounds as Dienophiles
CarrenÄo et al.11 have reported an enantioselective Diels-Alder reaction using chiral p-toluenesul®nyl-naphthoquinone as a dienophile. The reaction proceeded readily, yielding the product in about 50% de and 68±82% ee. The advantage of using sul®nyl compounds as dienophiles is the ease of subsequent removal of the sul®nyl group from the resulting compound by pyrolytic elimination. As shown in Scheme 5±12, an asymmetric Diels-Alder reaction using chiral sul®nyl compound 46 as the substrate results in product 48 in moderate to good enantiomeric excess after the subsequent removal of the sul®nyl group.
5.2CHIRAL DIENES
There are few reports about chiral auxiliary±attached diene components due, in part, to the di½culty of the preparation of the modi®ed dienes. Schemes 5±13,12 5±14,12b 5±15,13 and 5±1613 show some selected examples.
Scheme 5±13

278 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS



 n
n 


n 






Scheme 5±14
Scheme 5±15
Scheme 5±16
5.3DOUBLE ASYMMETRIC CYCLOADDITION
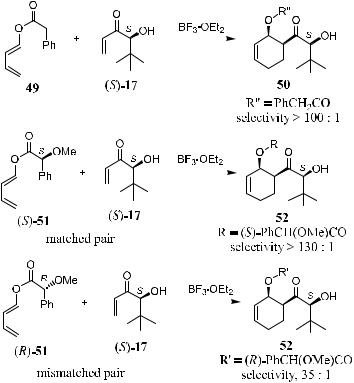
As mentioned in the previous section, chiral dienophile 17 is highly enantioselective and meets the criteria for double asymmetric induction. A set of asymmetric reactions have been performed by Masamune's group using 49 as an achiral diene and (S)/(R)-methyl mendelates (S)/(R)-51 as chiral dienes.2a The Diels-Alder reaction between (S)-17 and 49 exhibits the high diastereoselective potential of 17. As shown in Scheme 5±17, in the presence of a catalytic amount of BF3 Et2O, the reaction of (S)-17 and 49 gives compound 50 as the major product with a diastereoselectivity of over 100:1. The reaction of (S)-17 with
|
|
5.4 CHIRAL LEWIS ACID CATALYSTS |
279 |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 5±17
(S)-51 or (R)-51 in the presence of BF3 Et2O gives product 52 with a diastereoselectivity of 130:1 or 35:1, referring to the matched and mismatched pairs, respectively (Scheme 5±17).
5.4CHIRAL LEWIS ACID CATALYSTS
Perhaps the most attractive method of introducing enantioselectivity into the Diels-Alder reaction is to use a chiral catalyst in the form of a Lewis acidic metal complex. In recent years, this area has shown the greatest progress, with the introduction of many excellent catalytic processes. Quite a number of ligand±metal combinations have been evaluated for their potential as chiral catalysts in Diels-Alder reactions. The most commonly used metals are boron, titanium, and aluminum. Copper, magnesium, and lanthanides have also been used in asymmetric catalytic Diels-Alder reactions.
The most common sources of the chiral ligands employed for making a chiral Lewis acid complex are chiral diols with a C2-symmetric axis. This C2- symmetric feature reduces the number of competing transition states, which is

280 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
especially important when the sphere coordination number is greater than 4. Earlier examples14 of highly selective asymmetric induction in chiral Lewis acid±mediated Diels-Alder reactions were of this type. The main drawback of this type of promoter used to be its noncatalytic property. In other words, a stoichiometric amount of Lewis acid had to be used in the reaction. Nowadays, with modi®cations to the catalyst structure and the reaction procedure, the second generation of Lewis acids has been used in a catalytic manner, providing high yields and high enantioselectivities.15
5.4.1Narasaka's Catalyst
Narasaka et al.16 reported that 53 catalyzes Diels-Alder reactions of 54-type
Ê
substrates with diene in the presence of 4 A molecular sieves (Scheme 5±18). A remarkable solvent e¨ect on the enantioselectivity is observed. High enantioselectivity is attained using mesitylene as the solvent. As shown in Scheme 5±18, the reaction of 54a with isoprene proceeds smoothly in this solvent, a¨ording product 55a with 92% ee. Other 3-(3-substituted acryloyl)-1,3-oxazolidin-2-ones 54b±d also give good results (75±91% ee) when reacted with cyclopentadiene.
Scheme 5±18. Diels-Alder reaction of 54 with cyclopentadiene.
