
Principles and Applications of Asymmetric Synthesis
.pdf4.11 REFERENCES 261
been reported recently and shown some exciting results in the asymmetric synthesis of epoxide compounds from aldehydes.
Asymmetric epoxidation and the associated ring opening of epoxides, asymmetric dihydroxylation reactions, and asymmetric aminohydroxylation reactions have been extensively studied and widely used in the asymmetric synthesis of many intermediates or building blocks for important molecules. The study of its analogous aziridination reaction, however, is only at its primitive stage. Current e¨orts include seeking optimized reaction conditions, developing better chiral catalysts, and ®nding better nitrogen sources. These e¨orts are still ongoing, and their success will de®nitely open a new area for the asymmetric synthesis of amino group±containing compounds.
4.11REFERENCES
1.(a) Ewins, R. C.; Henbest, H. B.; McKervey, M. A. J. Chem. Soc. Chem. Commun. 1967, 1085. (b) Pirkle, W. H.; Rinaldi, P. L. J. Org. Chem. 1977, 42, 2080.
2.JuliaÂ, S.; Masana, J.; Vega, J. C. Angew. Chem. Int. Ed. Engl. 1980, 19, 929.
3.(a) Davis, F. A.; Harakal, M. E.; Awad, S. B. J. Am. Chem. Soc. 1983, 105, 3123.
(b)Davis, F. A.; Haque, M. S. J. Org. Chem. 1986, 51, 4083. (c) Davis, F. A.; Towson, J. C.; Weismiller, M. C.; Lal, S.; Carroll, P. J. J. Am. Chem. Soc. 1988, 110, 8477.
4.Yamada, S.; Mashiko, T.; Terashima, S. J. Am. Chem. Soc. 1977, 99, 1988.
5.Kagan, H. B.; Mimoun, H.; Mark, C.; Schurig, V. Angew. Chem. Int. Ed. Engl. 1979, 18, 485.
6.Michaelson, R. C.; Palermo, R. E.; Sharpless, K. B. J. Am. Chem. Soc. 1977, 99, 1990.
7.Katsuki, T.; Sharpless, K. B. J. Am. Chem. Soc. 1980, 102, 5974.
8.Martin, V. S.; Woodard, S. S.; Katsuki, T.; Yamada, Y.; Ikeda, M.; Sharpless, K. B. J. Am. Chem. Soc. 1981, 103, 6237.
9.Woodard, S. S.; Finn, M. G.; Sharpless, K. B. J. Am. Chem. Soc. 1991, 113, 106.
10.(a) Finn, M. G.; Sharpless, K. B. J. Am. Chem. Soc. 1991, 113, 113. (b) Potvin, P. G.; Bianchet, S. J. Org. Chem. 1992, 57, 6629.
11.Corey, E. J. J. Org. Chem. 1990, 55, 1693.
12.(a) Wang, Z. M.; Zhou, W. S.; Lin, G. Q. Tetrahedron Lett. 1985, 26, 6221.
(b)Wang, Z. M.; Zhou, W. S. Synth. Commun. 1989, 19, 2627.
13.Zhou, W. S.; Lu, Z.; Wang, Z. M. Tetrahedron Lett. 1991, 32, 1467.
14.Zhou, W. Wei, D. Tetrahedron Asymmetry 1991, 2, 767.
15.Gao, Y.; Hanson, R. M.; Klunder, J. M.; Ko, S. Y.; Masamune, H.; Sharpless, K. B. J. Am. Chem. Soc. 1987, 109, 5765.
16.Kragl, U.; Dreisbach, C. Angew. Chem. Int. Ed. Engl. 1996, 35, 642.
17.Canali, L.; Karjalainen, J. K.; Sherrington, D. C.; Hormi, O. J. Chem. Soc. Chem. Commun. 1997, 123.
262ASYMMETRIC OXIDATIONS
18.(a) Karjalainen, J. K.; Hormi, O.; Sherrington, D. C. Tetrahedron Asymmetry 1998, 9, 1563. (b) Karjalainen, J. K.; Hormi, O.; Sherrington, D. C. Tetrahedron Asymmetry 1998, 9, 2019.
19.(a) Rossiter, B. E.; Sharpless, K. B. J. Org. Chem. 1984, 49, 3707. (b) Ikegami, S.; Katsuki, T.; Yamaguchi, M. Chem. Lett. 1987, 83.
20.Karjalainen, J. K.; Hormi, O.; Sherrington, D. C. Tetrahedron Asymmetry 1998, 9, 3895.
21.Caron, M.; Sharpless, K. B. J. Org. Chem. 1985, 50, 1557.
22.Lin, G. Q.; Zeng, C. M. Chin. J. Chem. 1991, 9, 381.
23.Caron, M.; Carlier, P. R.; Sharpless, K. B. J. Org. Chem. 1988, 53, 5185.
24.Ti(OPri)2(N3)2 can be prepared in situ by stirring a mixture of Ti(OPri)4 ‡ 2 equivalents of Me3SiN3 in benzene under Ar or N2 for 5 hours until the solution becomes clear. A C-3 selective azide reagent consisting of NaN3 supported on a calcium zeolite has also been reported. Onaka, M.; Sugita, K.; Izumi, Y. Chem. Lett. 1986, 1327.
25.Benedetti, F.; Berti, F.; Norbedo, S. Tetrahedron Lett. 1998, 39, 7971.
26.Alvarez, E.; NunÄez, M. T.; MartõÂn, V. S. J. Org. Chem. 1990, 55, 3429.
27.Jung, M. E.; Jung, Y. H. Tetrahedron Lett. 1989, 30, 6637.
28.Bernet, B.; Vasella, A. Tetrahedron Lett. 1983, 24, 5491.
29.(a) Schmidt, R. R.; KlaÈger, R. Angew. Chem. Int. Ed. Engl. 1985, 24, 65.
(b)Tkaczuk, P.; Thornton, E. R. J. Org. Chem. 1981, 46, 4393.
30.Rama Rao, A. V.; Murali Dhar, T. G.; Chakraborty, T. K.; Gurjar, M. K.
Tetrahedron Lett. 1988, 29, 2069.
31.(a) Sharpless, K. B. J. Org. Chem. 1982, 47, 1378. (b) Viti, M. S. Tetrahedron Lett. 1982, 23, 4541.
32.Finan, J. M.; Kishi, Y. Tetrahedron Lett. 1982, 23, 2719.
33.Dai, L.; Lou, B.; Zhang, Y.; Guo, G. Tetrahedron Lett. 1986, 27, 4343.
34.Sajiki, H.; Hattori, K.; Hirota, K. Chem. Commun. 1999, 1041.
35.(a) Johnson, M. R.; Nakata, T.; Kishi, Y. Tetrahedron Lett. 1979, 4343. (b) Wood, R. D.; Ganem, B. Tetrahedron Lett. 1982, 23, 707.
36.Payne, G. B. J. Org. Chem. 1962, 27, 3819.
37.Katsuki, T.; Lee, A. W. M.; Ma, P.; Martin, V. S.; Sharpless, K. B.; Tuddenham, D.; Walker, F. J. J. Org. Chem. 1982, 47, 1373.
38.Masamune, S. J. Org. Chem. 1982, 47, 1375.
39.(a) Paterson, I.; Berrisford, D. J. Angew. Chem. Int. Ed. Engl. 1992, 31, 1179.
(b)SoÈdergren, M. J.; Andersson, P. G. J. Am. Chem. Soc. 1998, 120, 10760.
40.Iida, T.; Yamamoto, N.; Sasai, H.; Shibasaki, M. J. Am. Chem. Soc. 1997, 119, 4783.
41.Iida, T.; Yamamoto, N.; Matsunaga, S.; Woo, H.; Shibasaki, M. Angew. Chem. Int. Ed. Engl. 1998, 37, 2223.
42.Vogl, E. M.; Matsunaga, S.; Kanai, M.; Iida, T.; Shibasaki, M. Tetrahedron Lett. 1998, 39, 7917.
43.Wu, J.; Hou, X.; Dai, L.; Xia, L.; Tang, M. Tetrahedron Asymmetry 1998, 9, 3431.
44.Schaus, S. E.; Larrow, J. F.; Jacobsen, E. N. J. Org. Chem. 1997, 62, 4197.
4.11 REFERENCES 263
45.(a) Martinez, L. E.; Leighton, J. L.; Carsten, D. H.; Jacobsen, E. N. J. Am. Chem. Soc. 1995, 117, 5897. (b) Leighton, J. L.; Jacobsen, E. N. J. Org. Chem. 1996, 61, 389.
46.Konsler, R. G.; Karl, J.; Jacobsen, E. N. J. Am. Chem. Soc. 1998, 120, 10780.
47.(a) HaÈfele, B.; SchroÈter D.; JaÈger, V. Angew. Chem. Int. Ed. Engl. 1986, 25, 87. (b) Schreiber, S. L.; Schreiber, T. S.; Smith, D. B. J. Am. Chem. Soc. 1987, 109, 1525.
48.Okamoto, S.; Kobayashi, Y.; Kato, H.; Hori, K.; Takahashi, T.; Tsuji J.; Sato, F.
J.Org. Chem. 1988, 53, 5590.
49.Hatakeyama, S.; Sakurai, K.; Takano, S. Tetrahedron Lett. 1986, 27, 4485.
50.Kobayashi, Y.; Kato, N.; Shimazaki, T.; Sato, F. Tetrahedron Lett. 1988, 29, 6297.
51.For a review, see Kolb, H. C.; VanNieuwenhze, M. S.; Sharpless, K. B. Chem. Rev. 1994, 94, 2483.
52.Hofmann, K. A. Chem. Ber. 1912, 45, 3329.
53.Criegee, R.; Marchand, B.; Wannowius, H. Justus Liebigs. Ann. Chem. 1942, 550, 99.
54.Hentges, S. G.; Sharpless, K. B. J. Am. Chem. Soc. 1980, 102, 4263.
55.Jacobsen, E. N.; Marko, I.; Mungall, W. S.; SchroÈder, G.; Sharpless, K. B. J. Am. Chem. Soc. 1988, 110, 1968.
56.Lohray, B. B.; Kalantar, T. H.; Kim, B. M.; Park, C. Y.; Shibata, T.; Wai, J. S. M.; Sharpless, K. B. Tetrahedron Lett. 1989, 30, 2041.
57.Sharpless, K. B.; Amberg, W.; Bennani, Y. L.; Crispino, G. A.; Hartung, J.; Jeong,
K.S.; Kwong, H.; Morikawa, K.; Wang, Z.; Xu, D.; Zhang, X. J. Org. Chem. 1992, 57, 2768.
58.Crispino, G. A.; Jeong, K.; Kolb, H. C.; Wang, Z.; Xu, D.; Sharpless, K. B. J. Org. Chem. 1993, 58, 3785.
59.Becker, H.; Sharpless, K. B. Angew. Chem. Int. Ed. Engl. 1996, 35, 448.
60.Lohray, B. B.; Bhushan, V. Tetrahedron Lett. 1992, 33, 5113.
61.Lohray, B. B. Tetrahedron Asymmetry 1992, 3, 1317.
62.Yamada, T.; Narasaka, K. Chem. Lett. 1986, 131.
63.Tokles, M.; Snyder, J. K. Tetrahedron Lett. 1986, 27, 3951.
64.Tomioka, K.; Nakajima, M.; Koga, K. J. Am. Chem. Soc. 1987, 109, 6213.
65.Hirama, M.; Oishi, T.; Ito, S. J. Chem. Soc. Chem. Commun. 1989, 665.
66.Corey, E. J.; Jardine, P. D.; Virgil, S.; Yuen, P. W.; Connel, R. D. J. Am. Chem. Soc. 1989, 111, 9243.
67.(a) Shibata, T.; Gilheany, D. G.; Blackburn, B. K.; Sharpless, K. B. Tetrahedron Lett. 1990, 31, 3817. (b) Sharpless, K. B.; Amberg, W.; Beller, M.; Chen, H.; Hartung, J.; Kawanami, Y.; LuÈbben, D.; Manoury, E.; Ogino, Y.; Shibata, T.; Ukita, T. J. Org. Chem. 1991, 56, 4585.
68.Oishi, T.; Hirama, M. Tetrahedron Lett. 1992, 33, 639.
69.Hanessian, S.; Me¨re, P.; Girard, M.; Beaudoin, S.; Sanceau, J.; Bennani, Y.
J.Org. Chem. 1993, 58, 1991.
70.Wang, L.; Sharpless, K. B. J. Am. Chem. Soc. 1992, 114, 7568.
71.Oishi, T.; Hirama, M. J. Org. Chem. 1989, 54, 5834.
72.Turpin, J. A.; Weigel, L. O. Tetrahedron Lett. 1992, 33, 6563.
264ASYMMETRIC OXIDATIONS
73.Wang, Z.; Zhang, X.; Sharpless, K. B. Tetrahedron Lett. 1993, 34, 2267.
74.Tomioka, K.; Nakajima, M.; Koga, K. J. Chem. Soc. Chem. Commun. 1989, 1921.
75.(a) Sharpless, K. B.; Chong, A. O.; Oshima, K. J. Org. Chem. 1976, 41, 177.
(b)Herranz, E.; Sharpless, K. B. J. Org. Chem. 1978, 43, 2544.
76.BaÈckvall, J. E. Tetrahedron Lett. 1975, 2225.
77.(a) Li, G.; Chang, H. T.; Sharpless, K. B. Angew Chem. Int. Ed. Engl. 1996, 35, 451. (b) Rudolph, J.; Sennhenn, P. C.; Vlaar, C. P.; Sharpless, K. B. Angew. Chem. Int. Ed. Engl. 1996, 35, 2810.
78.Reiser, O. Angew. Chem. Int. Ed. Engl. 1996, 35, 1308.
79.(a) Li, G.; Angert, H. H.; Sharpless, K. B. Angew. Chem. Int. Ed. Engl. 1996, 35, 2813. (b) Reddy, K. L.; Sharpless, K. B. J. Am. Chem. Soc. 1998, 120, 1207. (c) O'Brien, P.; Osborne, S. A.: Parker, D. D. J. Chem. Soc. Perkin Trans. 1 1998, 2519.
80.O'Brien, P.; Osborne, S. A.; Parker, D. D. Tetrahedron Lett. 1998, 39, 4099.
81.Reddy, K. L.; Dress, K. R.; Sharpless, K. B. Tetrahedron Lett. 1998, 39, 3667.
82.(a) Bruncko, M.; Schlinglo¨, G.; Sharpless, K.B. Angew. Chem. Int. Ed. Engl. 1997, 36, 1483. (b) Li, G.; Sharpless, K. B. Acta Chem. Scand. 1996, 50, 649.
(c)Angelaud, R.; Landais, Y.; Schenk, K. Tetrahedron Lett. 1997, 38, 1407.
(d)Upadhya, T. T.; Sudalai, A. Tetrahedron Asymmetry 1997, 8, 3685. (e) Han, H.; Yoon, J.; Janda, K. D. J. Org. Chem. 1998, 63, 2045. (f ) Phukan, P.; Sudalai, A. Tetrahedron Asymmetry 1998, 9, 1001. (g) Cravotto, G.; Giovenzana, G. B.; Pagliarin, R.; Palmisano, G.; Sisti, M. Tetrahedron Asymmetry 1998, 9, 745.
83.(a) O'Brien, P. Angew. Chem. Int. Ed. Engl. 1999, 38, 326. (b) Goossen, L. J.; Liu, H.; Dress, K. R.; Sharpless, K. B. Angew. Chem. Int. Ed. Engl. 1999, 38, 1080.
84.Zhang, W.; Leobach, J. L.; Wilson, S. R.; Jacobsen E. N. J. Am. Chem. Soc. 1990, 112, 2801.
85.Irie, R.; Noda, K.; Ito, Y.; Matsumoto, N.; Katsuki, T. Tetrahedron Lett. 1990, 31, 7345.
86.(a) Lee, N. H.; Muci, A. R.; Jacobsen, E. N. Tetrahedron Lett. 1991, 32, 5055.
(b)Deng, L.; Jacobsen, E. N. J. Org. Chem. 1992, 57, 4320.
87.(a) Chang, S.; Lee, N. H.; Jacobsen, E. N. J. Org. Chem. 1993, 58, 6939. (b) Chang, S.; Galvin, J. M.; Jacobsen, E. N. J. Am. Chem. Soc. 1994, 116, 6937.
88.Brandes, B. D.; Jacobsen, E. N. J. Org. Chem. 1994, 59, 4378.
89.Palucki, M.; Pospisil, P. J.; Zhang, W.; Jacobsen, E. N. J. Am. Chem. Soc. 1994, 116, 9333.
90.Brandes, B. D.; Jacobsen, E. N. Tetrahedron Lett. 1995, 36, 5123.
91.Bolm, C. Angew. Chem. Int. Ed. Engl. 1991, 30, 403.
92.Hosoya, N.; Irie, R.; Katsuki, T. Synlett 1993, 261.
93.(a) Jacobsen, E. N.; Zhang, W.; GuÈler, M. L. J. Am. Chem. Soc. 1991, 113, 6703.
(b)Jacobsen, E. N.; Zhang, W.; Muci, A. R.; Ecker, J. R.; Deng, L. J. Am. Chem. Soc. 1991, 113, 7063. (c) Irie, R.; Noda, K.; Ito, Y.; Katsuki, T. Tetrahedron Lett. 1991, 32, 1055. (d) Chang, S.; Heid, R. M.; Jacobsen, E. N. Tetrahedron Lett. 1994, 35, 669. (e) Rychnovsky, S. D.; Hwang, K. Tetrahedron Lett. 1994, 35, 8927. (f ) PietikaÈinen, P. Tetrahedron Lett. 1995, 36, 319. (g) Zhang, W.; Jacobsen, E. N. J. Org. Chem. 1991, 56, 2296. (h) Larrow, J. F.; Jacobsen, E. N.; Gao, Y.; Hong,
4.11 REFERENCES 265
Y.; Nie, X.; Zepp, C. M. J. Org. Chem. 1994, 59, 1939. (i) Irie, R.; Noda, K.; Ito, Y.; Matsumoto, N.; Katsuki, T. Tetrahedron Asymmetry 1991, 2, 481. ( j) Collman,
J.P.; Zhang, X.; Lee, V. J.; U¨elman, E. S.; Brauman, J. I. Science 1993, 261, 1404. (k) Jacobsen, E. N.; Deng, L.; Furukawa, Y.; Martinez, L. E. Tetrahedron 1994, 50, 4323.
94.Ito, Y. N.; Katsuki, T. Tetrahedron Lett. 1998, 39, 4325.
95.Palucki, M.; Finney, N. S.; Pospisil, P. J.; GuÈler, M. L.; Ishida, T.; Jacobsen, E. N.
J.Am. Chem. Soc. 1998, 120, 948.
96.(a) Groves, J. T.; Viski, P. J. Org. Chem. 1990, 55, 3628. (b) Groves, J. T.; Myers,
R.S. J. Am. Chem. Soc. 1983, 105, 5971. (c) O'Malley, S.; Kodadek, T. J. Am. Chem. Soc. 1989, 111, 9116. (d) Collman, J. P.; Lee, V. J.; Zhang, X.; Ibers, J. A.; Brauman, J. I. J. Am. Chem. Soc. 1993, 115, 3834. (e) Mansuy, D.; Battioni, P.; Renaud, J.; Guerin, P. J. Chem. Soc. Chem. Commun. 1985, 155. (f ) Collman, J. P.; Zhang, X.; Lee, V. J.; Brauman, J. I.; J. Chem. Soc. Chem. Commun. 1992, 1647.
(g) Maillard, Ph.; Guerquin-Kern, J. L.; Momenteau, M. Tetrahedron Lett. 1991, 32, 4901. (h) Che, C.-M.; Yu, W.-Y. Pure Appl. Chem. 1999, 71, 281.
97.(a) Konishi, K.; Sugino, T.; Aida, T.; Inoue, S. J. Am. Chem. Soc. 1991, 113, 6487.
(b) Chiang, L.; Konishi, K.; Aida, T.; Inoue, S. J. Chem. Soc. Chem. Commun. 1992, 254. (c) Konishi, K.; Oda, K.; Nishida, K.; Aida, T.; Inoue, S. J. Am. Chem. Soc. 1992, 114, 1313.
98.(a) Naruta, Y.; Tani, F.; Maruyama, K. Chem. Lett. 1989, 1269. (b) Naruta, Y.; Tani, F.; Ishihara, N.; Maruyama, K. J. Am. Chem. Soc. 1991, 113, 6865. (c) Naruta, Y.; Ishihara, N.; Tani, F.; Maruyama, K. Chem. Lett. 1991, 1933. (d) Naruta, Y.; Tani, F.; Maruyama, K. Tetrahedron Lett. 1992, 33, 6323. (e) Naruta, Y.; Ishihara, N.; Tani, F.; Maruyama, K. Bull. Chem. Soc. Jpn. 1993, 66, 158.
99.Collman, J. P.; Wang, Z.; Straumanis, A.; Quelquejeu, M. J. Am. Chem. Soc. 1999, 121, 460.
100.Tu, Y.; Wang, Z.; Shi, Y. J. Am. Chem. Soc. 1996, 118, 9806.
101.Frohn, M.; Dalkiewicz, M.; Tu, Y.; Wang, Z.; Shi, Y. J. Org. Chem. 1998, 63, 2948.
102.Cao, G.; Wang, Z.; Tu, Y.; Shi, Y. Tetrahedron Lett. 1998, 38, 4425.
103.Wang, Z.; Shi, Y. J. Org. Chem. 1998, 63, 3099.
104.Miaskiewicz, K.; Smith, D. A. J. Am. Chem. Soc. 1998, 120, 1872.
105.(a) Yang, D.; Wang, X.; Wong, M.; Yip, Y.; Tang, M. J. Am. Chem. Soc. 1996, 118, 11311. (b) Yang, D.; Yip, Y.; Tang, M.; Wong, M.; Zheng, J.; Cheung, K.
J.Am. Chem. Soc. 1996, 118, 491.
106.Yang, D.; Wong, M.; Yip, Y.; Wang, X.; Tang, M.; Zheng, J.; Cheung, K. J. Am. Chem. Soc. 1998, 120, 5943.
107.For a review, see Li, A.; Dai, L.; Aggarwal, V. K. Chem. Rev. 1997, 97, 2341.
108.Aggarwal, V. K.; Ford, J. G.; Fonquerna, S.; Adams, H.; Jones, R. V. H.; Fieldhouse, R. J. Am. Chem. Soc. 1998, 120, 8328.
109.(a) Evans, D. A.; Takacs, J. M. Tetrahedron Lett. 1980, 21, 4233. (b) Davis, F. A.; Sheppard, A. C. Tetrahedron 1989, 45, 5703.
110.Evans, D. A.; Morrissey, M. M.; Dorow, R. L. J. Am. Chem. Soc. 1985, 107, 4346.
111.Davis, F. A.; Haque, M. S.; Ulatowski, T. G.; Towson, J. C. J. Org. Chem. 1986, 51, 2402.
266 ASYMMETRIC OXIDATIONS
112.(a) Davis, F. A.; Reddy, R. T.; Han, W.; Reddy, R. E. Pure Appl. Chem. 1993, 65, 633. (b) Davis, F. A.; Weismiller, M. C. J. Org. Chem. 1990, 55, 3715. (c) Davis, F. A.; Haque, M. S. J. Org. Chem. 1986, 51, 4083.
113.Zhu, Y.; Tu, Y.; Yu, H. W.; Shi, Y. Tetrahedron Lett. 1998, 39, 7819.
114.Tanner, D. Angew. Chem. Int. Ed. Engl. 1994, 33, 599.
115.Evans, D. A.; Woerpel, K. A.; Hinman, M. M.; Faul, M. M. J. Am. Chem. Soc. 1991, 113, 726.
116.Evans, D. A.; Faul, M. M.; Bilodeau, M. T.; Anderson, B. A.; Barnes, D. M.
J. Am. Chem. Soc. 1993, 115, 5328.
117.Li, Z.; Conser, K. R.; Jacobsen, E. N. J. Am. Chem. Soc. 1993, 115, 5326.
118.Minakata, S.; Ando, T.; Nishimura, M.; Ryu I.; Komatsu, M. Angew. Chem. Int. Ed. Engl. 1998, 37, 3392.
119.Jeong, J. U.; Tao, B.; Sagasser, I.; Henniges, H.; Sharpless, K. B. J. Am. Chem. Soc. 1998, 120, 6844.
120.(a) Groves, J. T.; Takahashi, T. J. Am. Chem. Soc. 1983, 105, 2073. (b) Mansuy, D.; Battioni, P.; Mahy, J. J. Am. Chem. Soc. 1982, 104, 4487. (c) Mahy, J.; Battioni, P.; Mansuy, D. J. Am. Chem. Soc. 1986, 108, 1079.
121. (a) Noda, K.; Hosoya, N.; Irie, R.; Ito, Y.; Katsuki, T. Synlett 1993, 469.
(b) Martres, M.; Gili, G.; Meou, A. Tetrahedron Lett. 1994, 35, 8787. (c) Tanner, D.; Andersson, P. G.; Harden, A.; Somfai, P. Tetrahedron Lett. 1994, 35, 4631.
(d)Zhang, W.; Lee, N. H.; Jacobsen, E. N. J. Am. Chem. Soc. 1994, 116, 425.
(e)Li, Z.; Quan, R. W.; Jacobsen, E. N. J. Am. Chem. Soc. 1995, 117, 5889. (f ) Nishikori, H.; Katsuki, T. Tetrahedron Lett. 1996, 37, 9245. (g) Lowenthal, R. E.; Masamune, S. Tetrahedron Lett. 1991, 32, 7373. (h) Harm, A. M.; Knight, J. G.; Stemp, G. Synlett 1996, 677. (i) Lai, T.; Kwong, H.; Che, C.; Peng, S. J. Chem. Soc. Chem. Commun. 1997, 2373. ( j) SoÈdergren, M. J.; Alonso, D. A.; Andersson, P. G. Tetrahedron Asymmetry 1997, 8, 3563. (k) Atkinson, R. S.; Gattrell, W. T.; Ayscough, A. P.; Raynham, T. M. J. Chem. Soc. Chem. Commun. 1996, 1935.
122.Lim, Y.; Lee, W. K. Tetrahedron Lett. 1995, 36, 8431.
123.Nakajima, K.; Tanaka, T.; Morita, K.; Okawa, K. Bull. Chem. Soc. Jpn. 1980, 53, 283.
124.Wipf, P.; Venkatraman, S.; Miller, C. P. Tetrahedron Lett. 1995, 36, 3639.
125.(a) Ploux, O.; Caruso, M.; Chassaing, G.; Marquet, A. J. Org. Chem. 1988, 53, 3154. (b) Nakajima, K.; Oda, H.; Okawa, K. Bull. Chem. Soc. Jpn. 1983, 56, 520.
126.Osborn, H.; Sweeney, J. B.; Howson, W. Tetrahedron Lett. 1994, 35, 2739.
127.Kim, D. Y.; Rhie, D. Y. Tetrahedron 1997, 53, 13603.
Principles and Applications of Asymmetric Synthesis
Guo-Qiang Lin, Yue-Ming Li, Albert S.C. Chan
Copyright ( 2001 John Wiley & Sons, Inc.
ISBNs: 0-471-40027-0 (Hardback); 0-471-22042-6 (Electronic)
 CHAPTER 5
CHAPTER 5
Asymmetric Diels-Alder and Other
Cyclization Reactions
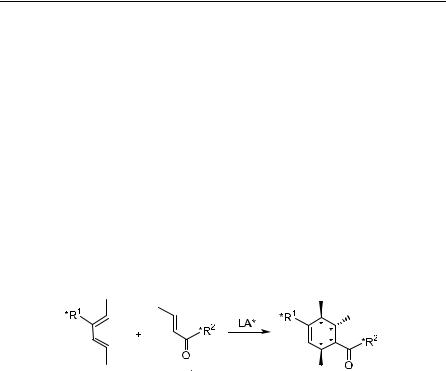
The Diels-Alder reaction is a powerful synthetic process for constructing complex molecules. The reaction has been extensively studied and re®ned since its discovery in 1928.1 The most attractive feature of the Diels-Alder reaction is its simultaneous, regioselective construction of two bonds, resulting in the creation of up to four chiral centers with largely predictable relative stereochemistry at the bond formation sites. Theoretically, there are a total of 24 ˆ 16 stereoisomers when atoms marked with an asterisk are all chiral centers (Scheme 5±1); therefore, the complete control of the reaction process to obtain enantiomerically pure products has been the object of active research in many laboratories.
Scheme 5±1
In addition to the syn-facial addition of the reaction, considerable advances have been made in achieving asymmetric induction through the following three methods: (1) attaching chiral auxiliaries to dienophiles, such as R 2 in Scheme 5±1; (2) attaching a chiral auxiliary to the diene, such as R 1 in Scheme 5±1; and (3) employing a chiral catalyst, usually a Lewis acid, such as LA* in Scheme 5±1. The ®rst and the second approaches have been the most commonly employed method for achieving asymmetric induction in the Diels-Alder reaction during the past decade. However, applying chiral catalytic Lewis acids has shown widespread utility, with several excellent catalysts readily available. Indeed, the search for e½cient chiral Lewis acids has been the prevailing issue in the study of asymmetric Diels-Alder reactions.
This chapter focuses on some typical examples, starting with the usual cycloaddition reactions and then the catalytic asymmetric Diels-Alder reactions, hetero Diels-Alder reactions, retro Diels-Alder reactions, and intramolecular
267

268 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Figure 5±1. De®nition of endoand exo-substituents in a bicyclic system.
Diels-Alder reactions. In the last two sections, the asymmetric 1,3-dipolar reaction [2‡3] and cyclopropanation reactions [1‡2] are discussed.
Before discussing asymmetric cycloaddition reactions, it is necessary to introduce the concepts of exo and endo. These are the stereochemical pre®xes that describe the relative con®gurations of a substituent on a bridged cyclic compound. (Note that they are applied only to the con®guration of substituents not on the bridge head.) Given a molecule where the two bridges that do not contain the substituent are of unequal length, the pre®x endo refers the substituent that is closer to the longer of the two unsubstituted bridges; and the pre®x exo refers to the substituent that is closer to the shorter bridge, as depicted in Figure 5±1.
5.1CHIRAL DIENOPHILES
Chiral dienophiles comprise the majority of asymmetric Diels-Alder reactions. As the most common chiral dienophiles, acrylates have been classi®ed into three categories, types I, II, and III (see Fig. 5±2). Type I reagents are chiral acrylates that incorporate the chiral group in a simple and straightforward manner. Type II reagents are those in which the chiral group is, in comparison with type I, one atom closer to the double bond. This type of compound typically requires more complex synthesis and the subsequent removal of the stereogenic center present in the compound. Furthermore, the recycling of the chiral group may be cumbersome. Type III reagents are acrylamide compounds bearing a chiral auxiliary connected via an amide linkage. This type of reagent exhibits high activity due to the positive electronic e¨ect at the nitrogen atom of the corresponding iminium salt.
Figure 5±2. Three types of chiral dienophiles.
5.1 CHIRAL DIENOPHILES |
269 |
5.1.1Acrylate
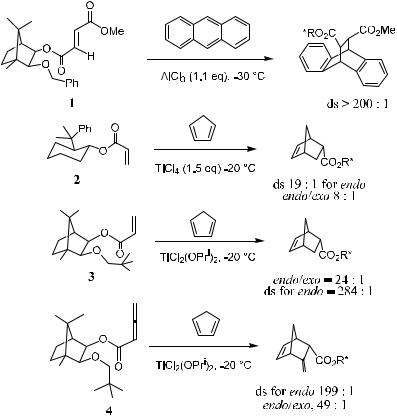
In general, compared with uncatalyzed cycloaddition reactions that may require a high temperature or high pressure, Lewis acid±catalyzed reactions can proceed at signi®cantly lower temperatures with high selectivity. Factors that improve the selectivity of catalyzed reactions generally include low temperature and a more organized coordination transition state between the Lewis acid and the substrate. Scheme 5±2 shows that, in the presence of a Lewis acid catalyst, chiral dienophiles 1±4 undergo a Diels-Alder reaction with a stereoselectivity as high as 200:1 or even better.2
For acrylates, or type I reagents, applied in asymmetric Diels-Alder reactions, several chiral auxiliaries such as menthol derivatives, camphor derivatives,1e,3 and oxazolidinones4 are available. Carbohydrate compounds have also been reported as chiral auxiliaries in a recent publication, although the stereoselectivity was not good.5 Here are examples in which asymmetric Diels-
Scheme 5±2

270 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Scheme 5±3 Retro synthetic analysis of the synthesis of sarkomycin.
Alder reactions involving type I reagents serve as a key step in the syntheses of important bioactive compounds.
The antibiotic sarkomycin (5), an antitumor agent, is a good target for synthetic e¨orts based on a Diels-Alder reaction. The retro synthesis of this compound is depicted in Scheme 5±3.
Linz et al.6 report the synthesis of enantiomerically pure cyclosarkomycin 6, a stable crystalline precursor of sarkomycin 5. As described in Scheme 5±3, 6 can be obtained from 8, an asymmetric Diels-Alder adduct of (E )-bromoacry- late. (E )-3-bromoacrylate 9a [the acrylate of (R)-pentolactone 11] and 9b [the acrylate of (S )-N-methyl hydroxyl succinimide 12] undergo TiCl4-mediated Diels-Alder reactions giving 10a or 10b0, the endo-product, with high diastereoselectivity (Scheme 5±4). With the key intermediate 10a in hand, synthesis of compound 6 is accomplished by following the reaction sequence shown in Scheme 5±5.
5.1.2a,b-Unsaturated Ketone
The design of type II substrates is based on the assumption that the chiral auxiliary R* may act as a highly e¨ective chiral promoter due to its proximity
Scheme 5±4
