
Principles and Applications of Asymmetric Synthesis
.pdf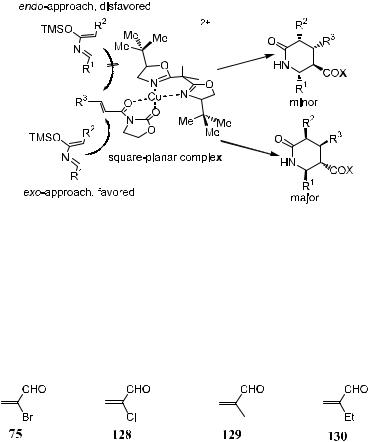
5.7 INTRAMOLECULAR DIELS-ALDER REACTIONS |
301 |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 5±9. Transition state for bis(oxazoline) catalyzed aza Diels-Alder reaction. Reprinted with permission by Am. Chem. Soc., Ref. 53.
5.6 FORMATION OF QUATERNARY STEREOCENTERS THROUGH DIELS-ALDER REACTIONS
Besides the methods discussed in Chapter 2, some quaternary stereocenters can also be conveniently constructed through the enantioselective Diels-Alder reaction of the 2-substituted acroleins 75 and 128±130.
In particular, Diels-Alder adducts from the enantioselective reaction of 2- bromo-acrolein and 2-chloroacrolein with a variety of dienes are of exceptional synthetic versatility. Readers are advised to consult the review article by Corey and Guzman-Perez.54 For the purpose of quick reference, chiral ligands commonly used in Diels-Alder reactions are listed in Table 5±6.
5.7INTRAMOLECULAR DIELS-ALDER REACTIONS
Intermolecular cycloadditions or Diels-Alder reactions have proved to be a successful route to several valuable intermediates for natural product syntheses. In creating new chiral centers, most of these reactions apply single asymmetric induction. As mentioned in Chapter 3, in the asymmetric synthesis of the octahydronaphthalene fragment, the Roush reaction is used twice. Subsequent intramolecular cyclization leads to the key intermediate, the aglycones, of several natural antitumor antibiotics. On the other hand, the Diels-Alder reaction of a dienophile-bearing chiral auxiliary can also be used intramolecularly to build

302 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
TABLE 5±6. List of Chiral Catalysts for the Diel-Alder Reaction
Chiral Catalyst |
References |
Chiral Catalyst |
References |
||
|
|
|
|
|
|
|
55 |
|
|
|
20, 21b |
|
|
|
|
|
|
56
57 |
58 |
38, 59 |
60 |
61 |
22, 61c, 62 |
28, 29, 30 |
63 |
60b
64
59a, 60b, 66
65
|
5.7 INTRAMOLECULAR DIELS-ALDER REACTIONS |
303 |
||||
TABLE 5±6 (Continued) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Chiral Catalyst |
References |
Chiral Catalyst |
References |
|||
|
|
|
|
|
|
|
|
60b |
|
|
|
39, 41 |
|
|
|
|
|
|
|
|
67
25a
68
59d
37a
69
70
30c
71
304 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Scheme 5±43
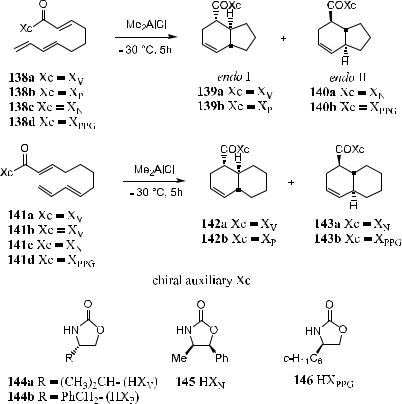
up important natural product subunits. In Scheme 5±43, Xc, which represents various chiral auxiliaries such as chiral amines, alcohols, or imides, can be removed afterward to complete the synthesis. In the given example, the bicyclic ring system is trans-fused. Table 5±7 gives some results for this type of intramolecular cyclization.4
Camphor sultam derivatives have proved to be e¨ective chiral auxiliaries in many di¨erent types of asymmetric reactions. As shown in Scheme 5±44, chiral camphor sulfam can be applied in the synthesis of …ÿ†-pulo'upone precursor 151 using an intramolecular Diels-Alder reaction. A Wittig reaction of 148 with 147 connects the chiral auxiliary to the substrate, and subsequent intramolecular Diels-Alder reaction via transition state 150 a¨ords product 151. Compound 151 already has the stereochemistry of …ÿ†-pulo'upone 153.72
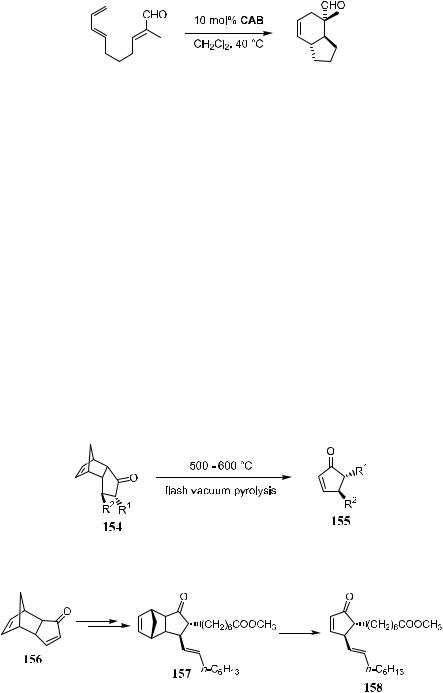
Apart from its application in intermolecular Diels-Alder reactions, chiral acyloxy boron (CAB) can also be used to e¨ect intramolecular Diels-Alder reactions with excellent stereoselectivity (Scheme 5±45).73

5.7 INTRAMOLECULAR DIELS-ALDER REACTIONS |
305 |
TABLE 5±7. Me2AlCl-Promoted Intramolecular Diels-Alder Reactions of Trienimides 138 and 141
Imide (Xc) |
endo I:endo II |
endo:exo |
Puri®ed Ratio |
Yield (%) |
|
|
|
|
|
|
|
138a |
(XV) |
83:17 |
>99:1 |
>99:1 |
60 (139a) |
138b |
(XP) |
95:5 |
>99:1 |
>99:1 |
73 (139b) |
138c (XN) |
15:85 |
>99:1 |
>99:1 |
70 (140a) |
|
138d |
(XPPG) |
3:97 |
>99:1 |
>99:1 |
65 (140a) |
141a |
(XV) |
92:8 |
>30:1 |
>99:1 |
65 (142a) |
141b |
(XP) |
97:3 |
>50:1 |
>99:1 |
88 (142b) |
141c (XN) |
9:91 |
>50:1 |
>99:1 |
70 (143a) |
|
141d |
(XPPG) |
6:94 |
>30:1 |
>99:1 |
70 (143b) |
Reprinted with permission by Am. Chem. Soc., Ref. 4.
Scheme 5±44
306 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Scheme 5±45
5.8RETRO DIELS-ALDER REACTIONS
The term Diels-Alder reaction in a general sense refers to the reaction between a diene and a dienophile. Retro Diels-Alder reaction is a process that, under certain conditions, produces diene and ole®n or a compound containing a CbC bond. The application of ¯ash vacuum pyrolysis to e¨ect the retro Diels-Alder reaction, as shown in Schemes 5±46 and 5±47, has become the standard procedure since the introduction of the method by Stork et al.74 in the 1970s. Therefore, alkenes that are di½cult to access by conventional methods may be obtained via retro Diels-Alder reactions.75 In particular, this reaction allows the preparation of thermodynamically less stable compounds such as 4,5-dialkyl cyclopenta-2-en-one. In this case, the alkene functional group can be regarded as being protected by cyclopentadiene (as shown in 154 or 157), which, after subsequent reaction, can easily be removed through quick pyrolysis.
Because compound 156 is a rigid framework that can be readily generated from a dimer of cyclopentadiene, this compound o¨ers an ideal structure on which many reactions can be carried out stereoselectively. Scheme 5±47 shows the synthesis of prostaglandin A1 or A2 (158). After the two side chains have
Scheme 5±46
Scheme 5±47

5.8 RETRO DIELS-ALDER REACTIONS |
307 |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 5±48
been attached to 156 to give compound 157 via a three-component coupling process,76 the desired compound 158 can be obtained after a quick pyrolysis.77 In the presence of a more reactive dienophile, a retro Diels-Alder reaction can be carried out at or below room temperature when catalyzed by a Lewis acid.78 In fact, this process can be regarded as a trans-Diels-Alder reaction in which the CbC bond is replaced by another more reactive functionality. Thus, when treated with fumaronitrile in the presence of EtAlCl2 at ambient temperature for 2 hours, compound 159 can easily be converted to compound 160 with
the removal of cyclopentadiene (Scheme 5±48).
Sometimes [4‡2] reversion of highly functionalized tricyclo[5.2.1.0.2.6]- decanones is not compatible with high temperature or Lewis acid treatment due to double bond isomerization, rearrangement, and/or extensive decomposition. Grieco and Abood79 applied pentamethylcyclopentadiene derivative 161 in replacement of 156 and found that [4‡2] reversion could be accelerated. Scheme 5±49 shows the application of this reagent in the synthesis of …‡†-(15S)- prostaglandin A2. Stirring a 0.06 M solution of 162 in ClCH2CH2Cl containing 3.5 equivalents of Me2AlCl and 5.0 equivalents of fumaronitrile at 10 C for over 48 hours gave 163 with 70% yield (Scheme 5±49).
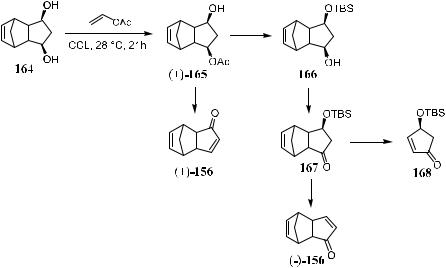
Compound 168 is a key intermediate for the synthesis of prostaglandin or prostacyclin compounds. Scheme 5±50 shows its preparation via a retro DielsAlder reaction and subsequent treatment. Using enzyme-catalyzed acetylation, Liu et al.80 succeeded in the asymmetric synthesis of enantiomerically pure …‡†/ …ÿ†-156 and …ÿ†-168 from the meso-diol 164. When treated with vinyl acetate, meso-diol 164 can be selectively acetylated to give …‡†-165 in the presence of Candida cyclindracea lipase (CCL). The yield for the reaction is 81%, and the enantiomeric excess of the product is 98.3%.
As previously mentioned, the optically pure synthons …‡†- and …ÿ†-156 can be used in the asymmetric synthesis of many important intermediates via
Scheme 5±49
308 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Scheme 5±50
retro Diels-Alder reactions. Indeed, …G†-chromomoric acid methyl ester, …G†- invictolide, and …G†-epiinvictolide have all been synthesized using …G†-156 as the starting material.81
5.9ASYMMETRIC DIPOLAR CYCLOADDITION
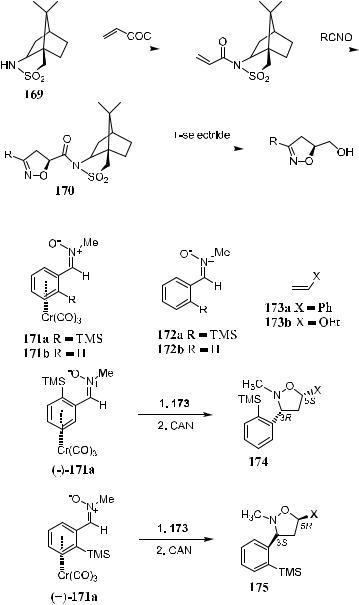
1,3-Dipolar addition is closely related to the Diels-Alder reaction, but allows the formation of ®ve-membered adducts, including cyclopentane derivatives. Like Diels-Alder reactions, 1,3-dipolar cycloaddition involves [4‡2] concerted reaction of a 1,3-dipolar species (the 4p component and a dipolar 2p component). Very often, condensation of chiral acrylates with nitrile oxides or nitrones gives only modest diastereoselectivity.82 1,3-Dipolar cycloaddition between nitrones and alkenes is most useful and convenient for the preparation of isoxazolidine derivatives, which can then be readily converted to 1,3-amino alcohol equivalents under mild conditions.83 The low selectivity of the 1,3-dipolar reaction can be overcome to some extent by introducing a chiral auxiliary to the substrate. As shown in Scheme 5±51, the reaction of 169 with acryloyl chloride connects the chiral sultam to the acrylic acid substrate, and subsequent cycloaddition yields product 170 with a diastereoselectivity of 90:10.84
Mukai et al.85 reported an asymmetric 1,3-dipolar cycloaddition of chromium(0)-complexed benzaldehyde derivatives. As shown in Scheme 5±52, heating chiral nitrone 171a, derived from Cr(CO)3-complexed benzaldehyde, with electron-rich ole®ns such as styrene (173a) or ethyl vinyl ether (173b) generates the corresponding chiral cis-3,5-disubstituted isoxazolidine adduct 174 or
5.9 ASYMMETRIC DIPOLAR CYCLOADDITION |
309 |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 5±51
Scheme 5±52
175 with ee in the range of 96±98%. These isoxazolidines can be further converted into chiral amino alcohols.
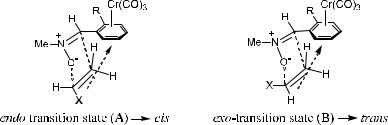
As compound 171 is a special planar chiral molecule, the dipolar reaction of compound 171 can be regarded as a substrate-controlled reaction. In 1,3-dipo-
310 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Figure 5±10
lar cycloaddition of the chromium-complexed nitrone [e.g., …ÿ†-171a or …‡†- 171a with electron-rich ole®ns 173a or 173b], the ortho-TMS group in the Cr complex governs the geometry of the aldehyde group, whereas the chromium complexation controls the facial selectivity. These two factors in the Cr complex seem to contribute to the observed high cis-stereoselectivity (Fig. 5±10). For example, when nitrone …‡†-171a is treated with styrene (173a), followed by decomplexation with cerium ammonium nitrate (CAN), the optically active cis- 3,5-disubstituted isoxazolidine …ÿ†-174 is produced with 65% yield. An asymmetric 1,3-dipolar cycloaddition of …‡†-171a with ethyl vinyl ether 173b also results in formation of an optically active isoxazolidine ring.
The high stereoselectivity can be explained by the proposed transition states (Fig. 5±10). The endo-transition state (A) o¨ers the advantage of releasing electrons through space from the electron-rich substituent on the dipolarophile to the electron-de®cient aromatic ring, thus resulting in a more stable (lower energy) transition state. The corresponding exo-transition state (B) does not have such a stabilizing factor. The high ee observed for 174 or 175 can best be explained by the approach of dipolarophiles to the chromium complexed nitrone, which must be from the face opposite that occupied by the Cr complexation. When uncomplexed nitrone 172a or 172b is considered, rather than (S)-171a or 171b, repulsion between the p-orbital electrons of the benzene ring and the electron-rich substituent on the dipolarophile in the endo-transition state (A) would become a strong destabilizing element. Preference for the endotype (A) over the exo-type (B) is therefore expected.
Using a stoichiometric amount of (R,R)-DIPT as the chiral auxiliary, optically active 2-isoxazolines can be obtained via asymmetric 1,3-dipolar addition of achiral allylic alcohols with nitrile oxides or nitrones bearing an electronwithdrawing group (Scheme 5±53).86a Furthermore, the catalytic 1,3-dipolar cycloaddition of nitrile oxide has been achieved by adding a small amount of 1,4-dioxane (Scheme 5±53, Eq. 3).86b The presence of ethereal compounds such as 1,4-dioxane is crucial for the reproducibly higher stereoselectivity.
Kobayashi's chiral lanthanide complex 63 has been used for asymmetric Diels-Alder reactions, and very good results have been obtained (see Section 5.4.2). This kind of complex is also e¨ective in asymmetric 1,3-dipolar reactions.87 The chiral ligand is prepared in situ by mixing Yb(OTf )3,
