
Principles and Applications of Asymmetric Synthesis
.pdf
8.1 ENZYMATIC AND RELATED PROCESSES |
453 |
kinetic resolution of chiral alcohols. Enzymes such as pig liver esterase (PLE), porcine pancreatic lipase (PPL), Pseudomonas sp. lipase, Candida cylindracea lipase, and Mucor mienei lipase are the more popular choices for these reactions.
In a lipase-catalyzed reaction, the acyl group of the ester is transferred to the hydroxyl group of the serine residue to form the acylated enzyme. The acyl group is then transferred to an external nucleophile with the return of the enzyme to its preacylated state to restart the catalytic cycle. A variety of nucleophiles can participate in this process. For example, reaction in the presence of water results in hydrolysis, reaction in alcohol results in esteri®cation or transesteri®cation, and reaction in amine results in amination. Kirchner et al.3 reported that it was possible to use hydrolytic enzymes under conditions of limited moisture to catalyze the formation of esters, and this is now becoming very popular for the resolution of alcohols.4
Kinetic resolution reactions on C2-symmetric substrates have important applications. Desymmetrization is just one example of such a kinetic resolution reaction. Enzymatic desymmetrization is outlined in Scheme 8±1.5,6
Scheme 8±1
A variety of enzymes (such as acetylcholine esterase, Porcine pancreatic lipase, Pseudomonas cepacia lipase, and Candida antarcita lipase) have been found useful in the preparation of enantiomerically pure cyclopentenol …‡†-2 from 1. The enantiomeric …ÿ†-2 has been prepared from diol 4 by enzymatic acetylation catalyzed by SP-345 with isopropenyl acetate in an organic medium. The key intermediate cyclopentanones …‡†-6, …ÿ†-6, 7, and 8, which are useful in the preparation of many bioactive molecules, can be obtained from 3 and 5 via routine chemical transformations.7


8.1 ENZYMATIC AND RELATED PROCESSES |
455 |
8.1.3Enantioselective Microbial Oxidation
It has been known since the 1950s that benzene and its derivatives can be oxidized to the corresponding cyclohexadienols in the presence of Pseudomonas putida (see Scheme 8±4 for an example).
Scheme 8±4. Oxidation of substituted benzene with Pseudomonas putida.
The Pseudomonas putidae organisms were initially selected for their ability to use benzene as the sole carbon source and were thereafter mutated to prevent further metabolism of the cyclohexadienols produced. This provides an e½cient approach to all four stereoisomers of sphingosine (Fig. 8±2)11 (see Chapter 3 for the synthesis of sphingosine compounds).
Figure 8±2. Preparation of sphingosine compounds via substituted benzene oxidation.
Bio-oxidation of bromobenzene 11 catalyzed by Pseudomonas putidae leads to diol 12. Protection of diol 12, followed by the addition of an acyl nitroso dienophile and subsequent reduction gives compound 14. This compound can be used as the key intermediate in the preparation of …‡†-1-deoxy-galacto- nojirimycin (16) and related indolizidine compounds (15) (Scheme 8±5).12
Another extensively studied bio-oxidation is the monooxygenase-catalyzed Baeyer-Villiger oxidation of cyclic ketones.13 As the ®rst example, cyclohexanone monooxygenase from Acinetobacter sp. NCIB 9871 was expressed in baker's yeast to create a general purpose reagent for asymmetric BaeyerVilliger oxidation. This engineered yeast combines the advantages of puri®ed enzymes with the bene®ts of whole-cell reactions. It has been used to oxidize a series of 2-, 3-, and 4-substituted cyclohexanones, providing the corresponding

456 ENZYMATIC REACTIONS AND MISCELLANEOUS ASYMMETRIC SYNTHESES
Scheme 8±5
TABLE 8±1. Yeast-Mediated Kinetic Resolution of 2-Substituted Ketones via BaeyerVilliger Oxidation
Entry |
R |
Yield (%) |
ee (%) |
Yield (%) |
ee (%) |
|
|
|
|
|
|
1 |
Me |
50 |
49 |
Ð |
Ð |
2 |
Et |
79 |
95 |
69 |
98 |
3 |
n-Pr |
54 |
97 |
66 |
92 |
4 |
i-Pr |
41 |
98 |
46 |
96 |
5 |
Allyl |
59 |
98 |
58 |
98 |
6 |
n-Bu |
59 |
98 |
64 |
98 |
ee ˆ Enantiomeric excess.
Reprinted with permission by Am. Chem. Soc., Ref. 13c.
lactones with good yields and high ee. Table 8±1 shows typical results for asymmetric Baeyer-Villiger oxidation of 2-substituted cyclohexanones.
It is only recently that isolated enzymes have been used in the presence of appropriate cofactor recycling systems.14 Not long ago, application of the whole-cell system was the only way to get high yields and high ee in enzymecatalyzed organic synthesis.
8.1.4Formation of C±C Bond
Cyanohydrins are starting materials of widespread interest for preparing important compounds such as a-hydroxy acids/esters, a-amino acids, b-amino alcohols, a-hydroxy aldehydes, vicinal diols, and a-hydroxy ketones. Cyanohydrin compounds can be synthesized using various chiral catalysts such as cyclic

8.1 ENZYMATIC AND RELATED PROCESSES |
457 |
dipeptides15 and chiral complexes bearing Ti,16 Al,17 or B18 as the central metal. The enantioselective synthesis of cyanohydrins has also been achieved by using enzymes, hydronitrilases (oxynitrilases), isolated from di¨erent plant sources.19
Oxynitrilases, isolated from either almond [(R)-speci®c]20 or a microorganism [(S )-speci®c],21 catalyze the enantioselective addition of cyanide ion to a range of aromatic or aliphatic aldehydes, providing cyanohydrins with ee values of up to 99%.
Scheme 8±6
To suppress the noncatalyzed reaction (which decreases the enantioselectivity) between acetone cyanohydrin and the substrate, ethyl acetate is required as a co-solvent, and a low reaction temperature is also essential. Han et al.22 found that in organic solution with a trace amount of water the above reaction proceeds with the same high enantioselectivity as in the presence of an aqueous bu¨er. The reaction can be carried out at a wide range of temperatures from 0 to 30 C. To avoid using highly toxic potassium or sodium cyanide, acetone cyanohydrin is used as a cyano donor.
The following enzymes are useful for asymmetric organic synthesis:
PLE, pig liver esterase PPL, pig pancreatic lipase
CCL, Candida cylindracae lipase a-chym, a-chymotrypsin from bovine p-lipase, from Pseudomonas species PS lipase
A lipase AF±Z MY DF 360
AnL, Aspergilus niger PrL, Penicillinm reguerferti CIL, Candida lypolytica
CSL, Candida sp 382
For discussions of some representative enzyme-catalyzed reactions, see elsewhere.23

458 ENZYMATIC REACTIONS AND MISCELLANEOUS ASYMMETRIC SYNTHESES
8.1.5Biocatalysts from Cultured Plant Cells
In recent years, extensive attention has been focused on ®nding cultured plant cells that can be used as catalysts for organic functional group transformations. A number of transformations employing freely suspended or immobilized plant cell cultures have been reported.24 For example, Akakabe et al.25 report that immobilized cells of Daucus carota from carrot can be used to reduce prochiral carbonyl substrates such as keto esters, aromatic ketones, and heterocyclic ketones to the corresponding secondary alcohols in (S)-con®guration with enantiomeric excess of 52±99% and chemical yields of 30±63%.
Other kinds of plant cell cultures such as immobilized tobacco cells have also been studied for the analogous transformation. The results show that plant cell cultures provide an accessible way of converting several prochiral ketones into the corresponding chiral secondary alcohols with reasonable chemical yield and high enantioselectivity.
8.2MISCELLANEOUS METHODS
Recently, an increasing number of methods have become known in organic synthesis that are not easy to compile into an appropriate place in previous sections. They are hence grouped in this section.
8.2.1 Asymmetric Synthesis Catalyzed by Chiral Ferrocenylphosphine Complex
Chiral ferrocenylphosphine ligand26 such as 17 bears a pendant side chain and a hydroxy group at the terminal position. When compound 17 is used in the palladium-catalyzed asymmetric allylic amination of allylic substrates containing a 1,3-disubstituted propenyl structure, product with good to excellent enantiomeric excess can be obtained (Scheme 8±7). Catalyst prepared in situ from Pd(dba)2 and (R)- or (S)-17 catalyzes the amination of substrate 18 with high yield and up to 97% ee. The proposed transition state of the active catalyst is shown in Figure 8±3. The hydroxyl group in the ligand can interact with the nucleophile. This facilitates its attack on the substrate and promotes the reaction.
Both enantiomers of racemic 2-propenyl acetate can be formed from mesotype p-alkyl palladium intermediates by oxidative addition. p-Allylpalladium complexes with two alkyl substituents at the 1- and 3-positons are known to
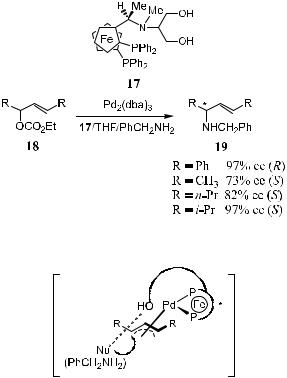
8.2 MISCELLANEOUS METHODS |
459 |
Scheme 8±7
Figure 8±3. Transition state of 17 catalyzed reaction.
adopt the syn-conformation of both substituents. The attack by the nucleophile takes place from the preferred side on either of the two diastereotopic p-alkyl carbon atoms in the p-allyl palladium intermediate. The (E )-geometry amination product is ®nally formed with high selectivity.
8.2.2Asymmetric Hydrosilylation of Ole®ns
Catalytic asymmetric hydrosilylation of prochiral ole®ns has become an interesting area in synthetic organic chemistry since the ®rst successful conversion of alkyl-substituted terminal ole®ns to optically active secondary alcohols (>94% ee) by palladium-catalyzed asymmetric hydrosilylation in the presence of chiral monodentate phosphine ligand (MOP, 20). The introduced silyl group can be converted to alcohol via oxidative cleavage of the carbon±silicon bond (Scheme 8±8).27
When norbornene is treated with trichlorosilane in this manner, quantitative yield of exo-2-trichlorosilylnorbornane is obtained, and (1S,2S,4R)-exo-2- norbornanol can be obtained in 96% ee upon hydrogen peroxide oxidation.28 This reaction can be extended to other ole®nic substrates such as 2,5-

460 ENZYMATIC REACTIONS AND MISCELLANEOUS ASYMMETRIC SYNTHESES
Scheme 8±8
dihydrofuran. As shown in Scheme 8±9, the catalytic asymmetric hydrosilylation of 2,5-dihydrofuran with trichlorosilane in the presence 0.1 mol% of (R)-MOP (20)±coordinated palladium complex yields the corresponding hydrosilylation product 22 in up to 95% ee (Scheme 8±9).29
Scheme 8±9
Other metal complexes such as titanium or ruthenium complexes can also be used to catalyze the ole®n hydrosilylation reactions. Further information is provided elsewhere.30
8.2.3Synthesis of Chiral Biaryls
The hindered biaryls are examples of a di¨erent type of chiral compound due to their rotational restriction around a C±C single bond. As long as the orthosubstituents in a compound such as 23 are bulky enough, the compound can exist in two forms, 23 and its enantiomer 230, which are not interconvertible.

8.2 MISCELLANEOUS METHODS |
461 |
Compounds with chirality caused by restriction of the C±C axial covalent bond include a range of naturally occurring as well as synthesized compounds. Not only do axially chiral biaryls constitute a structural feature of many products, but using biaryls possessing C2 symmetry as chiral ligands in asymmetric synthesis has also been an area of considerable activity. Indeed, the synthesis of these axial chiral biaryls has gradually become one of the most interesting subjects in asymmetric organic synthesis. Here are some examples of these chiral biaryl compounds31:
In most cases, construction of axial nonracemic biaryls can be realized by metal-mediated intramolecular biaryl coupling in which the chirality is induced by ortho-substituted auxiliaries in an aromatic system.
Preparation of the chiral biphenyls and binaphthyls with high enantioselectivity can be achieved via substitution of an aromatic methoxyl group with an aryl Grignard reagent using oxazoline as the chiral auxiliary.38 Schemes 8±10 and 8±11 outline the asymmetric synthesis of such chiral biaryl compounds.
As shown in Scheme 8±11, nucleophilic entry from the a-face (24a) may be hindered by the sterically bulky substituent R2 on the oxazoline moiety; therefore entry from the b-face 24b predominates. Free rotation of the magnesium methoxy bromide may be responsible for the sense of the axial chirality formed in the biaryl product. If the azaenolate intermediate 25 is re-aromatized with a 20-methoxy substituent complexed to Mg, (S)-biphenyl product is obtained. Upon re-aromatization of azaenolate 25B, (R)-product is obtained.
Another method for synthesizing chiral biaryls is the substrate-controlled

462 ENZYMATIC REACTIONS AND MISCELLANEOUS ASYMMETRIC SYNTHESES
Scheme 8±10
Scheme 8±11
asymmetric Ullmann reaction. Scheme 8±12 depicts the asymmetric synthesis of enantiomerically pure C2-symmetric binaphthyls.39
When subjected to Ullmann biaryl reactions, 8-bromo-1-oxazolinylnaph- thalene (S)-27 can be converted to the 8,80-substituted binaphthyl 28 in high diastereomeric excess (another isomer, the (aR,S)-diastereomer, is less than 3%).40
