
Principles and Applications of Asymmetric Synthesis
.pdf
5.5 HETERO DIELS-ALDER REACTIONS |
291 |
Scheme 5±29
In the course of asymmetric hetero Diels-Alder reaction studies, Maruoka et al.38 developed a new chiral organometallic catalyst of type 90. These catalysts are applicable to various reactions of siloxydienes and aldehydes, promoting high enantioselectivity. For example, treating benzaldehyde with siloxydiene in the presence of (R)-90 (Ar ˆ Ph, 10 mol%) at ÿ20 C for 2 hours yields cis-dihydropyrane 92 (77%) and its trans-isomer (7%) after exposing the adduct to tri¯uoroacetic acid (Scheme 5±29). A variety of siloxydienes have been treated with aldehydes in the same manner, yielding adducts in moderate to high enantiomeric excess (Table 5±4).
TABLE 5±4. (R)-90 Catalyzed Diels-Alder Reaction
Aldehyde |
Diene |
(R)-92 |
Product |
Yield (%) |
ee (%, con®g.) |
|
|
|
|
|
|
|
|
c-C6H11CHO |
91 |
Ar ˆ Ph |
|
65 |
91 |
(2S,3R) |
PhCHO |
93a |
Ar ˆ Ph |
|
71 |
67 |
(R) |
ee ˆ Enantiomeric excess.
Reprinted with permission by Am. Chem. Soc., Ref. 38.

292 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Scheme 5±30
Li et al.39 reported the hetero Diels-Alder reaction of alkyl-3-(t- butyldimethylsilyl) oxy-1,3-butadiene 95 with ethyl glyoxylate 96 in the presence of a salen±Co(II) catalyst 94 (2 mol%). Product 97 was obtained in 75% isolated yield with an endo:exo ratio >99:1. The enantiomeric excess of the endo-form was up to 52% (Scheme 5±30).
Upon treatment with n-Bu4NF, product 97 is converted to 98, which is a key intermediate for the synthesis of the monosaccharide 3-deoxy-d-manno-2- octulosonic acid (KDO)Ðan essential constituent of the outer membrane lipopolysaccharide (LPS) of gram-negative bacteria.40
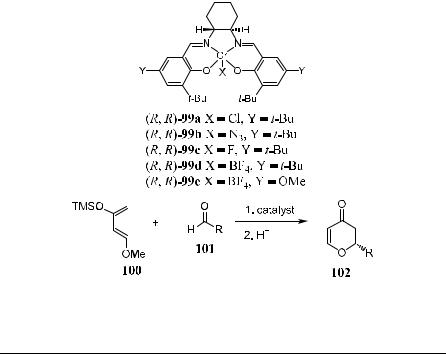
Schaus et al.41 have also reported an asymmetric hetero Diels-Alder reaction of Danishefsky's diene 10042 with aldehyde 101 catalyzed by chromium(III) complex 99 bearing a similar chiral salen ligand. Product 102 is obtained in moderate to good yield and stereoselectivity (Scheme 5±31 and Table 5±5).
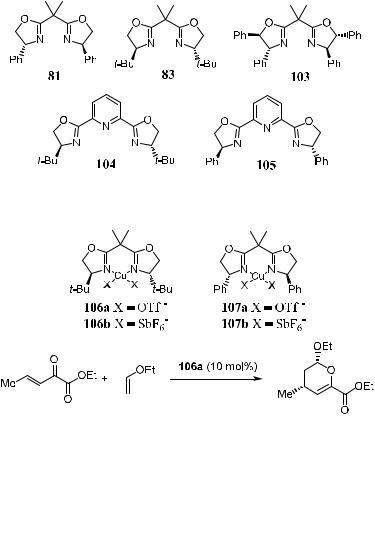
The C2-symmetric bis(oxazoline)±Cu(II) complexes have proved to be very e¨ective in asymmetric aldol reactions (see Section 3.4.3), as well as Diels-Alder reactions (see Section 5.4.6). These compounds are also powerful catalysts in hetero Diels-Alder reactions. Figure 5±8 shows some of the bis(oxazoline) ligands applied in asymmetric hetero Diels-Alder reactions.
Evans et al.43 and Thorhauge et al.44 report that hetero Diels-Alder reactions in the presence of 81 or 83 coordinated copper complex proceed smoothly, resulting in excellent yields and enantiomeric excess.
Jùrgensen's group44a carried out the reaction using the anhydrous form of chiral bis(oxazoline) coordinated copper complex. Complex 106 containing 83 as the chiral ligand was found to be the most e¨ective. As shown in Scheme 5±32, the asymmetric hetero Diels-Alder reaction of b,g-unsaturated a-keto esters with acyclic enol ethers results in products with excellent yield and enantioselectivity.
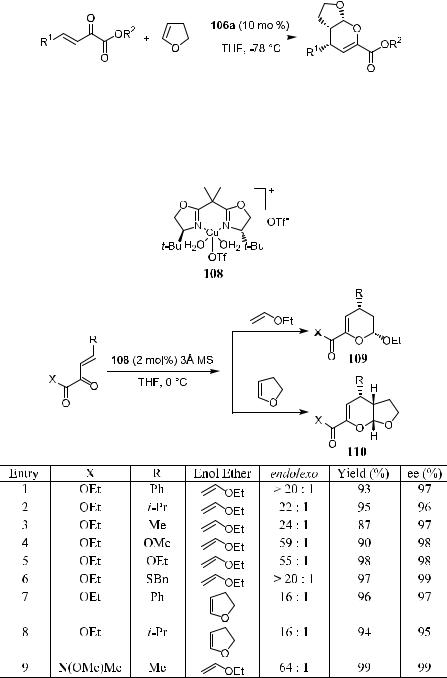
This catalyst is also e¨ective with cyclic enol ethers.44a As shown in Scheme
5.5 HETERO DIELS-ALDER REACTIONS |
293 |
Scheme 5±31
TABLE 5±5. Hetero Diels-Alder Reaction Catalyzed by 99
|
|
|
|
ee (%) |
|
|
|
|
|
Entry |
R in Aldehyde |
Catalyst 99d |
Catalyst 99e |
|
|
|
|
|
|
1 |
Ph |
87 |
65 |
|
2 |
C6H11 |
93 |
85 |
|
3 |
n-C5H11 |
83 |
62 |
|
4 |
2-Furyl |
76(99) |
68 |
|
5 |
(E )-PhCHbCH |
70 |
73(99) |
|
6 |
p-BrC6H4CH2OCH2 |
79 |
84(99) |
|
7 |
o-ClC6H4COOCH2 |
83(99) |
72 |
|
ee ˆ Enantiomeric excess.
Reprinted with permission by Am. Chem. Soc., Ref. 41.
5±33, the hetero Diels-Alder reaction of b,g-unsaturated a-keto ester with cyclic enol ether dihydrofuran also gives high stereoselectivity.
The procedure reported by Jùrgensen's group used anhydrous copper complex. The complex is hygroscopic, and special attention must be paid to handling the catalyst, as well as when carrying out the reaction. Evans' group43a found that the hetero Diels-Alder reaction can proceed as well using the hydrate of complex 108 as the catalyst in the presence of molecular sieves. This greatly simpli®es the reaction. As shown in Scheme 5±34, the asymmetric hetero Diels-Alder reaction of acyclic or cyclic enol ethers with b,g-unsaturated
294 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Figure 5±8. Bis(oxazoline) ligands used in asymmetric hetero Diels-Alder reactions.
Scheme 5±32. Reprinted with permission by Wiley-VCH Verlag Germany, Ref. 44(a).
keto esters generates product 109 or 110 in excellent yield and with excellent diastereoselectivity as well as enantioselectivity.
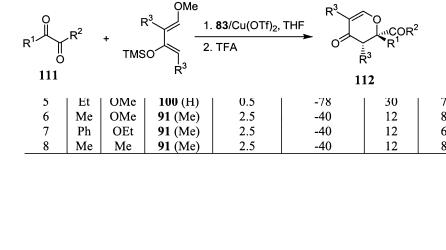
The hetero Diels-Alder reactions discussed thus far use 2±10 mol% of catalyst. Jùrgensen's group44b found that the reaction could be carried out even at very low catalyst loading. The catalyst can conveniently be prepared in situ by mixing the chiral ligand 83 and copper tri¯ate in the reaction system. Scheme 5±35 shows that product 112 can be obtained with good yield and high enan-
5.5 HETERO DIELS-ALDER REACTIONS |
295 |
||
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 5±33. Reprinted with permission by Wiley-VCH Verlag Germany, Ref. 44(a).
Scheme 5±34. Reprinted with permission by Wiley-VCH Verlag Germany, Ref. 43a.
296 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Scheme 5±35. Reprinted with permission by Am. Chem. Soc., Ref. 44b.
tiomeric excess after treating the hetero Diels-Alder reaction product with TFA.
The copper complex of these bis(oxazoline) compounds can also be used for hetero Diels-Alder reactions of acyl phosphonates with enol ethers.43b A favorable acyl phosphonate±catalyst association is achieved via complexation between the vicinal CbO and PbO functional groups. The acyl phosphonates are activated, leading to facile cycloaddition with electron-rich alkenes such as enol ethers. The product cyclic enol phosphonates can be used as building blocks in the asymmetric synthesis of complicated molecules. Scheme 5±36 shows the results of such reactions.
5.5.2Aza Diels-Alder Reactions
In Section 5.5.1, we discussed the oxo hetero Diels-Alder reaction, or the hetero Diels-Alder reaction involving oxygen as the hetero atom. There is another type of hetero Diels-Alder reaction in which nitrogen-containing compounds are involved, and these are referred to as aza Diels-Alder reactions.
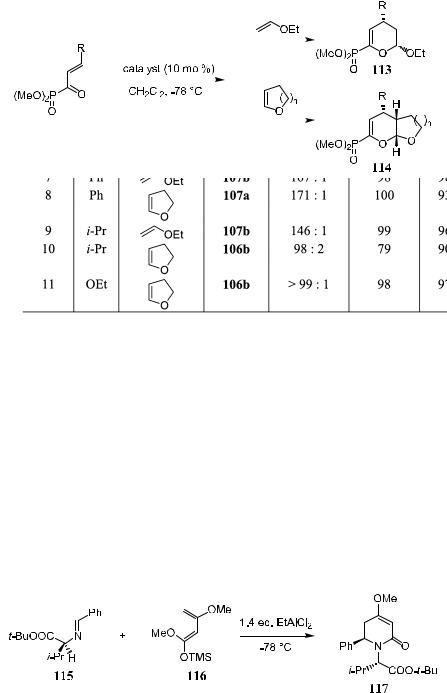
Asymmetric aza Diels-Alder reactions provide a useful route to optically active heterocyclics such as piperidines and tetrahydroquinolines.45 Although successful examples of diastereoselective approaches had been reported as early as 10 years ago,46 only recently have enantioselective reactions been accomplished.47 For example, the reaction of chiral amine-derived aromatic imine 115 with Brassard's diene 116 gives adduct 117 with up to 95% diastereoselectivity (Scheme 5±37).48
A stoichiometric amount of BINOL±boron chiral Lewis acid 118 activates
|
|
|
5.5 HETERO DIELS-ALDER REACTIONS |
297 |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 5±36. Reprinted with permission by Am. Chem. Soc., Ref. 43b.
Scheme 5±37

298 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Scheme 5±38
the reaction of aromatic imine 119 with Danishefsky's diene 100, a¨ording adduct 120 in up to 90% ee (Scheme 5±38).49
In the presence of a catalytic amount of Lewis acid, chiral esters 121 and 122 show improved diastereoselectivity. When they are allowed to react with cyclopentadiene, adducts of type 123 are obtained with good stereoselectivity.50
Kobayashi et al.51 have reported an asymmetric Mannich-type reaction using chiral zirconium catalysts of type 124 (see Section 3.7). This catalyst is also e¨ective for asymmetric aza Diels-Alder reactions. Kobayashi's study showed that the ligand had a profound in¯uence on the yields and enantioselectivities of the reaction, and NMI (1-methylimidazole) proved to be the best ligand.51 With an increase in the amount of catalyst, both the chemical yields and enantioselectivities of the product can be enhanced. Scheme 5±39 depicts such aza Diels-Alder reactions, and its table shows that good to excellent enantioselectivity can be obtained for most reactions.

5.5 HETERO DIELS-ALDER REACTIONS |
299 |
|
|
|
|
Scheme 5±39. Reprinted with permission by Wiley-VCH Verlag Germany, Ref. 51.
Scheme 5±40. Reprinted with permission by Wiley-VCH Verlag GmbH, Ref. 52.
Jùrgensen's group reported the aza Diels-Alder reactions in the presence of several chiral catalysts.52 They found that chiral bis(oxazoline) ligands 81, 83, 103, 104, and 105, which were e¨ective in asymmetric oxo hetero Diels-Alder reactions, induced the aza Diels-Alder reaction of a-imino ester with Danishefsky's diene with only poor to moderate enantioselectivity. Selected results are listed in Scheme 5±40.
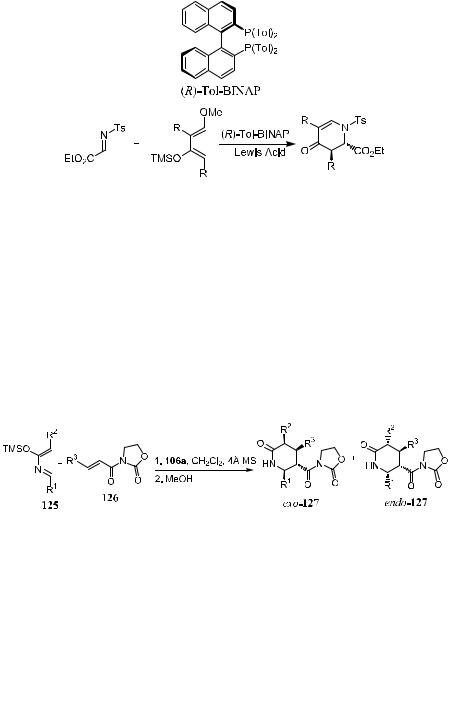
On the other hand, the combination of Tol±BINAP with CuClO4 has been shown to be very e¨ective for aza Diels-Alder reactions. As shown in Scheme 5±41, moderate yield and good to excellent enantioselectivity can be obtained in the reaction of a-imino ester with diene 91 or 100.
Another successful aza Diels-Alder reaction involves 2-azadienes of type 125 with dienophile 126 in the presence of bis(oxazoline) catalyst 106a.53 Product 127 is obtained in a high exo:endo ratio, as well as high enantiomeric excess for the exo-isomer of 127. The high enantioselectivity and high yield rely on the chiral catalyst 106a, which activates the dienophile by complexation with an appropriate functional group and does not irreversibly coordinate with the
300 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Scheme 5±41. Reprinted with permission by Wiley-VCH Verlag GmbH, Ref. 52.
nucleophilic nitrogen atom of the azadiene. The experimental results are shown in Scheme 5±42.
The high stereoselectivity of the reaction can be explained by the transition state shown in Figure 5±9. An exo-approach of the diene to the less hindered face of the square±planar complex causes the high enantioselectivity.
Scheme 5±42. Reprinted with permission by Am. Chem. Soc., Ref. 53.
