
Principles and Applications of Asymmetric Synthesis
.pdf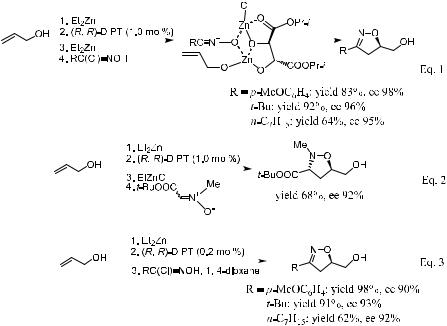
|
|
|
|
|
|
|
|
5.9 ASYMMETRIC DIPOLAR CYCLOADDITION |
311 |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 5±53
(S)-BINOL and a tertiary amine together. The reaction proceeds smoothly at room temperature to a¨ord the corresponding isoxazolidine derivatives with good yield and diastereoselectivity, as well as enantioselectivity. Kobayashi's study shows that combining the chirality of BINOL and the chiral amine is crucial for getting better enantioselectivity. Use of N,N-di-[(1R)-(a-naphthyl)ethyl]- N-methylamine [176, (R)-MNEA] resulted in the highest enantioselectivity. As shown in Scheme 5±54, in the presence of chiral ytterbium tri¯ate complex 63 containing (R)-MNEA 176 as the chiral tertiary amine, the asymmetric 1,3- dipolar reaction of nitrone 177 with dienophile 178 proceeds smoothly, providing endo-product 179 in excellent yield and diastereoselectivity as well as enantiomeric excess.
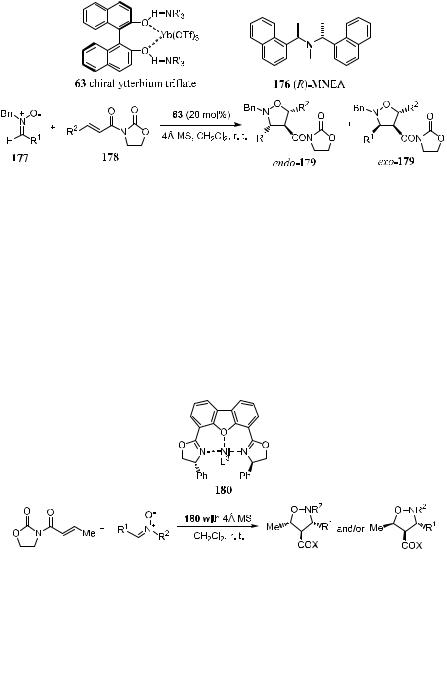
Bis(oxazoline)-type complexes, which have been found useful for asymmetric aldol reactions, Diels-Alder, and hetero Diels-Alder reactions can also be used for inducing 1,3-dipolar reactions. Chiral nickel complex 180, which can be prepared by reacting equimolar amounts of Ni(ClO)4 6H2O and the corresponding (R,R)-4,6-dibenzofurandiyl-2,20-bis(4-phenyloxazoline) (DBFOX/Ph) in dichloromethane, can be used for highly endo-selective and enantioselective
Ê
asymmetric nitrone cycloaddition. The presence of 4 A molecular sieves is essential to attain high selectivities.88 In the absence of molecular sieves, both the diastereoselectivity and enantioselectivity will be lower. Representative results are shown in Scheme 5±55.
312 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Scheme 5±54. Reprinted with permission by Am. Chem. Soc., Ref. 87.
Scheme 5±55. Reprinted with permission by Am. Chem. Soc., Ref. 88.

5.10 ASYMMETRIC CYCLOPROPANATION |
313 |
5.10ASYMMETRIC CYCLOPROPANATION
Cyclopropane subunit has been found in many natural and synthetic compounds possessing important biological properties. For example, the two compounds FR-90084889 and U-10630590 show strong bioactivity.
Furthermore, cyclopropane structures have often served as intermediates in organic synthesis. For these reasons, ole®n cyclopropanation has proved to be a useful tool for synthetic organic chemists. This has led to the development of several methods for cyclopropanation reactions,91 including the metalcatalyzed reactions of diazo compounds with ole®ns, as well as the SimmonsSmith reaction.
Taking 1,2-disubstituted cyclopropane as an example, retro synthesis analysis shows that there are three possible ways to disconnect the three-membered ringÐa, b, and c as shown in Figure 5±11. Route a involves the addition of methylene across a double bond, and this is often a stereospeci®c conversion or Simmons-Smith reaction.92 One can clearly see that route b or c will encounter the issue of cis/trans-product formation.
Two strategies have been adopted for asymmetric cyclopropanation. First, there are auxiliary-based methods, involving a covalently attached adjacent chiral moiety on either the ole®n or the cyclopropylating agent. The second process, on the other hand, employs a chiral ligand on a metal catalyst. This method is more applicable to route b or c, and this is an issue that warrants further discussion.
Figure 5±11. Asymmetric cyclopropanation.
314 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Scheme 5±56
5.10.1 Transition Metal Complex±Catalyzed Cyclopropanations
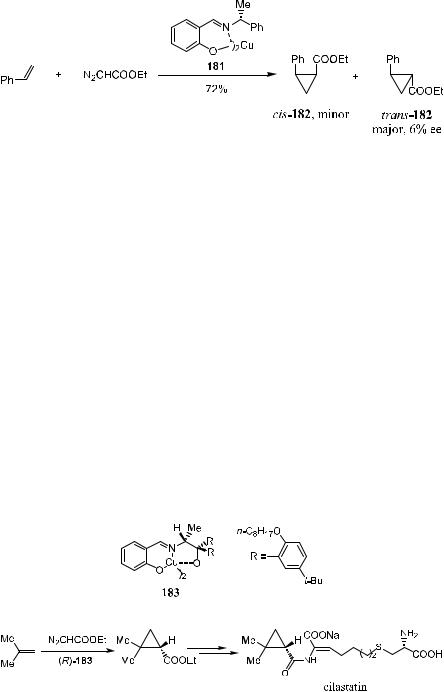
Certain transition metal complexes catalyze the decomposition of diazo compounds. The metal-bonded carbene intermediates behave di¨erently from the free species generated via photolysis or thermolysis of the corresponding carbene precursor. The ®rst catalytic asymmetric cyclopropanation reaction was reported in 1966 when Nozaki et al.93 showed that the cyclopropane compound trans-182 was obtained as the major product from the cyclopropanation of styrene with diazoacetate with an ee value of 6% (Scheme 5±56). This reaction was e¨ected by a copper(II) complex 181 that bears a salicyladimine ligand.
This report initiated activities that resulted in the discovery of 183, the Aratani catalyst. It is now applied in the enantioselective synthesis of ethyl-2,3- dimethoxycyclopropanecarboxylate, the key intermediate in the preparation of cilastatin (Scheme 5±57).94
The next major contribution in asymmetric cyclopropanation was the introduction of chiral semicorrin ligands 184 by Fritschi et al.95 This ligand has been used for coordinating with copper and has been found to provide improved enantiocontrol in the cyclopropanation of monosubstituted ole®ns. Copper(I), coordinated by only one semicorrin ligand, is believed96 to be the catalytically active oxidation state. The copper(I) oxidation state can be reached directly
Scheme 5±57

5.10 ASYMMETRIC CYCLOPROPANATION |
315 |
Scheme 5±58
Figure 5±12. Chiral bis(oxazoline) ligands for asymmetric cyclopropanation.
through the reduction of Cu(II)L2 by the diazo compound or by the treatment of copper(II)±semicorrin with phenylhydrazine (Scheme 5±58).
Subsequently, Lowenthal and co-workers,31a,97 Evans et al.,31b and MuÈller et al.98 reported chiral bis(oxazoline) ligands 185, 186, and 83 as shown in Figure 5±12. The gem-dimethyl [(bis)oxazoline] 83±coordinated copper catalyst is the most widely used ligand. The catalyst is prepared in situ by mixing ligand 83 with an equal molar amount of CuOTf. Asymmetric cyclopropanation of isobutylene with ethyl diazoacetate (EDA) gives ethyl 2,3-dimethylcyclopro- pane carboxylate with >99% ee.
Unlike the catalytic epoxidation or aziridination reactions of simple alkenes, where enantiocontrol is the only stereochemical di¨erentiation, synthetically e¨ective intermolecular cyclopropanation requires both diastereocontrol and enantiocontrol. High diastereoselectivity for the trans-isomer can be achieved with the use of bulky diazoacetates such as BDA99 187 or DCM97 188.
For example, the asymmetric cyclopropanation of styrene with BDA catalyzed by CuOTf±83 yields cyclopropane with a trans:cis ratio of 94:6 and 99%
eefor the trans-isomer.31
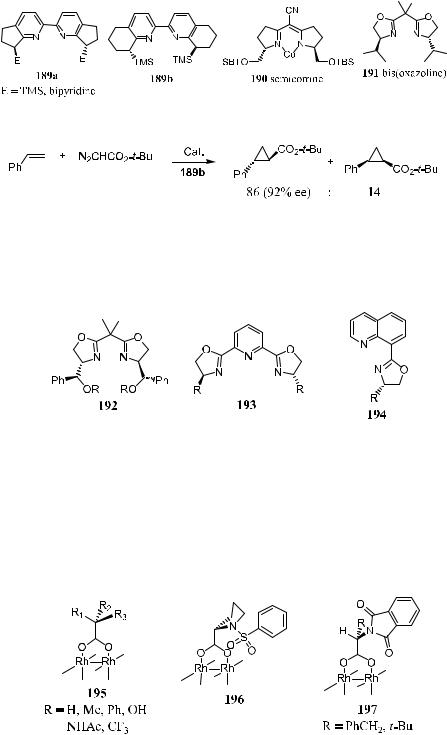
Ito and Katsuki100 report that compounds 189a±191 can also be used as
chiral ligands in asymmetric cyclopropanation reactions and that the bipyridine ligand has a potential for catalyzing the reaction (see Fig. 5±13 for the structure of these chiral ligands). Scheme 5±59 shows one example of using bipyridine compound 189b as a chiral ligand in the cyclopropanation reaction of styrene with t-butyl diazoacetate; 92% ee for the trans-product can be obtained.
316 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Figure 5±13. Chiral ligands for asymmetric cyclopropanation.
Scheme 5±59
In addition to the above ligands 189±191, bis(oxazolinyl)pyridine compounds 192±194 have also been applied in asymmetric cyclopropanation reactions, but only moderate enantioselectivity has been achieved.101
Use of chiral semicorrins and related nitrogen heterocyclic compounds as chiral ligands in asymmetric catalysis has been reviewed periodically. Interested readers are referred to the review by Pfaltz.102
Chiral dirhodium(II) catalysts with carboxylate or carboxamidate ligands have recently been developed to take advantage of their versatility in metal carbene transformation, and these have now become the catalysts of choice for cyclopropanation. Chiral carboxylate ligands 195,103 196,104 and 197105 have been used for tetrasubstitution around a dirhodium(II) core. However, the enantioselectivity in intermolecular reactions with simple ketenes is marginal.

5.10 ASYMMETRIC CYCLOPROPANATION |
317 |
Chiral carboxamidate-ligated dirhohdium(II) compounds 198,106 199,107 200,108 and 201109 are not oxygen sensitive and possess a long shelf life. Although their initial application to intermolecular cyclopropanation reactions between styrene and l- or d-menthyl diazoacetate provided lower stereocontrol than that of the Aratani catalyst, they have proved to be the most e¨ective catalysts for intramolecular cyclopropanation reactions of diazoacetate and diazoacetamide.110 These compounds can also be used to induce asymmetric cyclopropenation reactions.111
Intramolecular cyclopropanation has a noteworthy advantage. Unlike intermolecular asymmetric cyclopropanation, the intramolecular reaction produces only one diastereomer due to geometric constrains on the fused bicyclic products. Doyle has extensively studied the intramolecular enantioselective reactions of a variety of alkenyl diazoacetates catalyzed by chiral rhodium carboxamides 198 and 200 and has achieved excellent results.
In the simplest case, the reaction of allyl diazoacetate, the catalyst (S)-198 or (R)-198 in a concentration as low as 0.1 mol% can still catalyze the formation of enantiomeric-3-oxabicyclo[3.1.0]hexan-2-ones with 95% ee (Scheme 5±60). Substituted alkyl diazoacetates undergo intramolecular cyclopropanation, with similarly high enantiomeric excess (Scheme 5±61).110
Scheme 5±60
Scheme 5±61
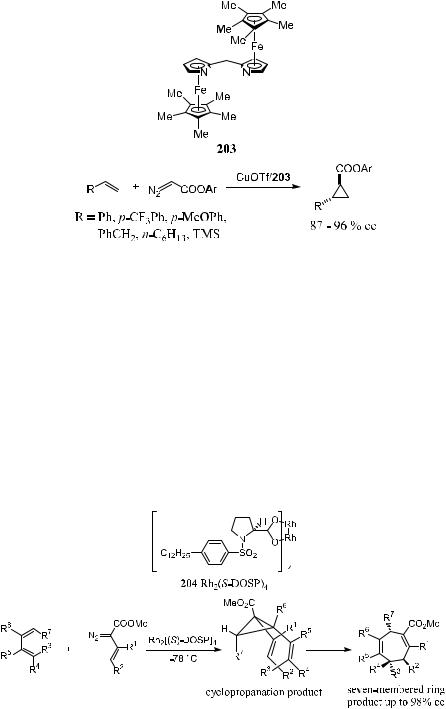
318 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Scheme 5±62
Lo and Fu112 have reported a new type of planar±chiral ligand 203 for the enantioselective cyclopropanation of ole®ns. As shown in Scheme 5±62, asymmetric cyclopropanation in the presence of chiral ligand 203 proceeds smoothly, giving the cyclopropanation product with high diastereoselectivity and enantioselectivity.
The Cope rearrangement of divinyl cyclopropanes is an attractive method for the stereoselective synthesis of seven-membered rings.113 Due to the requirement of a boat transition state for this Cope rearrangement, multiple stereogenic centers can be formed in a well-de®ned manner. As shown in Scheme 5±63, compound rhodium(II) N-dodecylbenzenesulfonyl prolinate (204) catalyzes the asymmetric cyclopropanation. Subsequent Cope rearrangement gives the corresponding seven-membered ring compound 1,4-cycloheptadiene
Scheme 5±63

5.10 ASYMMETRIC CYCLOPROPANATION |
319 |
with high yield and stereoselectivity.114 This example illustrates the beauty of tandem reactions.
5.10.2The Catalytic Asymmetric Simmons-Smith Reaction
Among methods of preparing optically active cyclopropane compounds, the Simmons-Smith reaction, ®rst reported in 1958, is of signi®cance. This reaction refers to the cyclopropanation of alkene with a reagent prepared in situ from a zinc±copper alloy and diiodomethane. The reaction is stereospeci®c with respect to the geometry of the alkene and is generally free from side reactions in contrast to reactions involving free carbenes.
In the Simmons-Smith reaction, the purpose of copper is simply to activate the Zn surface and has no other role. Iodomethyl zinc behaves as a weak nucleophile. As generally expected, the presence of an allylic oxygen gives a large rate enhancement, and more remote neighboring oxygen atoms also in¯uence the stereochemical course of the reaction.
The reaction was ®rst carried out with the substrate bearing the chiral auxiliary. Scheme 5±64 shows the asymmetric cyclopropanation reaction using 2,4- pentandiol as a chiral auxiliary.115 Scheme 5±65 illustrates the use of optically pure 1,2-trans-cyclohexanediol as a chiral auxiliary in asymmetric SimmonsSmith cyclopropanation.116 Excellent yield and diastereoselectivity are obtained in most cases.
Scheme 5±64
Scheme 5±65. Reprinted with permission by Pergamon Press Ltd., Ref. 116.
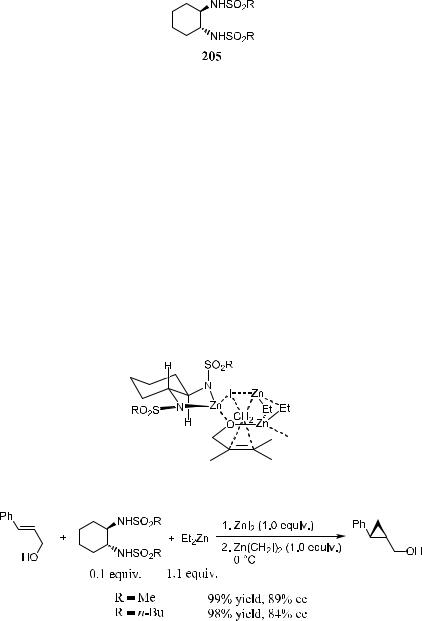
320 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Ukaji et al.117 reported an enantioselective cyclopropanation reaction in which moderate enantiomeric excess was obtained when a stoichiometric amount of diethyl tartrate was used as a chiral modi®er. Takahashi et al.118 achieved better results using the C2-symmetric chiral disulfonamide 205 as the chiral ligand.
Figure 5±14 illustrates the transition state in the reaction. The free hydroxyl group is necessary for producing an e¨ective chiral environment, probably through complexation as a zinc alkoxide.118
Scheme 5±66 shows another example of chiral bis(sulfonamide) 205± catalyzed asymmetric cyclopropanation of allylic alcohol.119
Another chiral ligand that can be used in asymmetric Simmons-Smith reactions to build up a cyclopropane moiety is the dioxaborolane compound 206, containing tartaric acid diamide as a chiral ligand.120 This compound is an e½cient chiral controller for the enantioselective conversion of allylic alcohols to substituted cyclopropylmethanols with both high yields and high enantiomeric excesses.121 The design of this dioxaborolane compound relies on the presence of an acidic (boron) and a basic (amide) site that allow the simultaneous complexation of the acidic halomethylzinc reagent and the basic allylic metal alkoxide. A wide range of substrates such as allylic alcohols, polyenes, 2,4-dien-1-ols, homoallylic alcohols, and allylic amines can then undergo cyclopropanation reactions.120
Figure 5±14. Transition State for 205 Catalyzed Cyclopropanation.
Scheme 5±66
