
Principles and Applications of Asymmetric Synthesis
.pdf
|
5.4 CHIRAL LEWIS ACID CATALYSTS |
281 |
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 5±19. Asymmetric synthesis of …‡†-paniculide A.
This type of chiral diol has received considerable attention as a chiral ligand not only in Diels-Alder reactions but also in other reactions such as alkylation and allylation of carbonyl groups, mainly because of its ease of preparation from tartrate. Yamamoto and Narasaka17 described the application of this chiral titanium catalyst in the synthesis of …‡†-paniculide A (62), a highly oxidized sesquiterpene. Scheme 5±19 outlines the synthesis route to this compound. The enantioselective cycloaddition of [(E )-3-(3-(5,5-dimethyl-1,3-dioxaborinan-2- yl)propenoyl)]-1,3-oxazolidin-2-one (56) to 1-acetoxy-3-methyl-1,3-butadiene (57) is a key step. The 3-hydroxyl group in 56 is masked as its borate and can be regenerated by m-CPBA oxidation of the Diels-Alder reaction product 58. Highly enantiomerically pure product 59 can be obtained with the desired stereochemistry of …‡†-paniculide A.
The Lewis acid catalyst 53 is now referred to as the Narasaka catalyst. This catalyst can be generated in situ from the reaction of dichlorodiisopropoxytitanium and a diol chiral ligand derived from tartaric acid. This compound can also catalyze [2‡2] cycloaddition reactions with high enantioselectivity. For example, as depicted in Scheme 5±20, in the reaction of alkenes bearing alkylthio groups (ketene dithioacetals, alkenyl sul®des, and alkynyl sul®des) with electron-de®cient ole®ns, the corresponding cyclobutane or methylenecyclobutene derivatives can be obtained in high enantiomeric excess.18
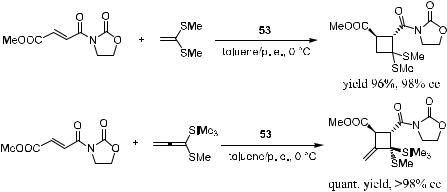
282 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Scheme 5±20
5.4.2Chiral Lanthanide Catalyst
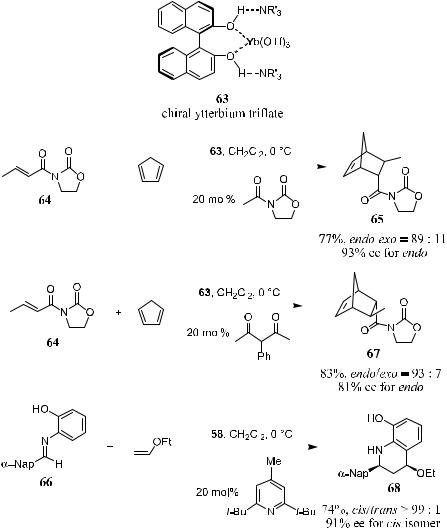
In the presence of a catalytic amount of chiral lanthanide tri¯ate 63, the reaction of 3-acyl-1,3-oxazolidin-2-ones with cyclopentadiene produces Diels-Alder adducts in high yields and high ee. The chiral lanthanide tri¯ate 63 can be prepared from ytterbium tri¯ate, (R)-…‡†-binaphthol, and a tertiary amine. Both enantiomers of the cycloaddition product can be prepared via this chiral lanthanide(III) complex±catalyzed reaction using the same chiral source [(R)- …‡†-binaphthol] and an appropriately selected achiral ligand. This achiral ligand serves as an additive to stabilize the catalyst in the sense of preventing the catalyst from aging. Asymmetric catalytic aza Diels-Alder reactions can also be carried out successfully under these conditions (Scheme 5±21).19
5.4.3Bissulfonamides (Corey's Catalyst)
Sulfonamide derivatives of a-amino acids and the similar bissulfonamide derivatives of diamines can be used to prepare reactive Lewis acid complexes. Corey20 reported the Lewis acid (R,R)- or (S,S)-complex 69, which can be employed at 10 mol% level to catalyze the Diels-Alder reaction of cyclopentadiene and imide. Reactions catalyzed by this complex give an endo:exo ratio of over 50:1, as well as a high ee (91%) at ÿ78 C, and this can be further improved to 95% by carrying out the reaction at ÿ90 C.20 The related aluminum complex 69b shows very similar reactivity at ÿ78 C, with generally higher ee values, typically over 95%, for the reaction of cyclopentadiene derivatives with imide.20,21
Thus, as shown in Scheme 5±22,20 the reaction of 71 with cyclopentadiene in the presence of 10 mol% of (S,S)-69b at ÿ90 C generates 72a at 92% yield with 95% ee (endo:exo > 50:1). The corresponding reaction of 64b with cyclopentadiene gives 72b at 88% yield with 94% ee (endo:exo ˆ 96:4). The reaction of 71 with 70b in the presence of 0.1 mol% of (S,S)-69b at ÿ78 C gives the adduct 72c at 94% yield with 95% ee.
|
|
|
|
|
5.4 CHIRAL LEWIS ACID CATALYSTS |
283 |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 5±21
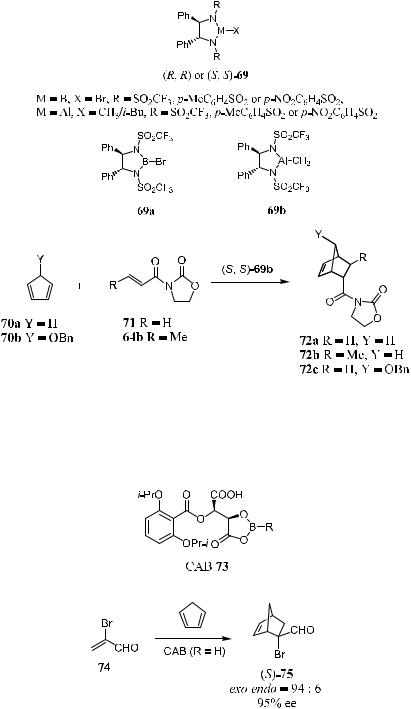
5.4.4Chiral Acyloxy Borane Catalysts
Stable aryl boronates derived from tartaric acid catalyze the reaction of cyclopentadiene with vinyl aldehyde with high selectivity. Chiral acyloxy borane (CAB), derived from tartaric acid, has proved to be a very powerful catalyst for the enantioselective Diels-Alder reaction and hetero Diels-Alder reaction. Scheme 5±23 presents an example of a CAB 73 (R ˆ H) catalyzed Diels-Alder reaction of a-bromo-a,b-enal 74 with cyclopentadiene. The reaction product is another important intermediate for prostaglandin synthesis. In the presence of
284 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Scheme 5±22
Scheme 5±23
5.4 CHIRAL LEWIS ACID CATALYSTS |
285 |
10 mol% of 73 (R ˆ H), the reaction of 74 with cyclopentadiene proceeds smoothly, giving (S)-75 at a quantitative yield and with 95% ee.22
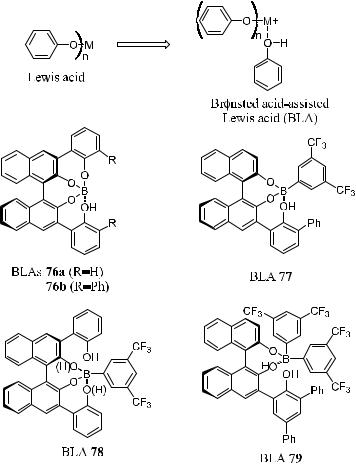
5.4.5Brùnsted Acid±Assisted Chiral Lewis Acid Catalysts
Lewis acids of chiral metal aryloxides prepared from metal reagents and optically active binaphthol derivatives have played a signi®cant role in asymmetric synthesis and have been extensively studied.23 However, in Diels-Alder reactions, the asymmetric induction with chiral metal aryloxides is, in most cases, controlled by steric interaction between a dienophile and a chiral ligand. This kind of interaction is sometimes insu½cient to provide a high level of enantioselectivity.
In recent years, a number of groups (e.g., Hawkins and Loren,24 Corey and
Figure 5±5. Brùnsted acid-assisted lewis acids.

286 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
TABLE 5±2. Enantioselective Diels-Alder Reaction of Various a-Substituted a,b-Enals with Cyclopentadiene Catalyzed by (R)-BLA 76a
Entry |
Dienophile |
Yield (%) (exo:endo) |
ee (%) (con®g.) |
||
|
|
|
|
|
|
1 |
a-Bromoacrolein |
>99 |
(>99:1) |
99 |
(S) |
2 |
CH2 bCMeCHO |
>99 |
(>99:1) |
99 |
(R) |
3 |
CH2 bCEtCHO |
>99 |
(97:3) |
92 |
|
4 |
(E )-MeCHbCHMeCHO |
>99 |
(>99:1) |
98 |
|
5 |
1-Formylcyclopentene |
>99 |
(98:2) |
93 |
|
6 |
CH2 bCHCHO |
91 |
(9:91) |
40 |
(R) |
7 |
CH2 bCHCHO |
85 |
(14:86) |
92 |
(R)a |
8 |
(E )-MeCHbCHCHO |
12 |
(11:89) |
36 |
(R) |
ee ˆ Enantiomeric excess. a (R)-76b was used instead of (R)-76a.
Reprinted with permission by Am. Chem. Soc., Ref. 27.
Lo,25 and Ishihara et al.26) have reported that the attractive p±p donor±acceptor interaction between a dienophile and a chiral ligand is highly e¨ective for inducing asymmetry. Encouraged by this ®nding, Ishihara et al.27 developed Brùnsted acid±assisted chiral Lewis acid (BLA) catalysts 76±79 (Fig. 5±5). This new class of catalysts can promote highly enantioselective Diels-Alder reactions through a combination of intramolecular hydrogen bonding and the attractive p±p donor±acceptor interaction of the hydroxyl aromatic groups in the chiral Lewis acids in the transition state assembly.
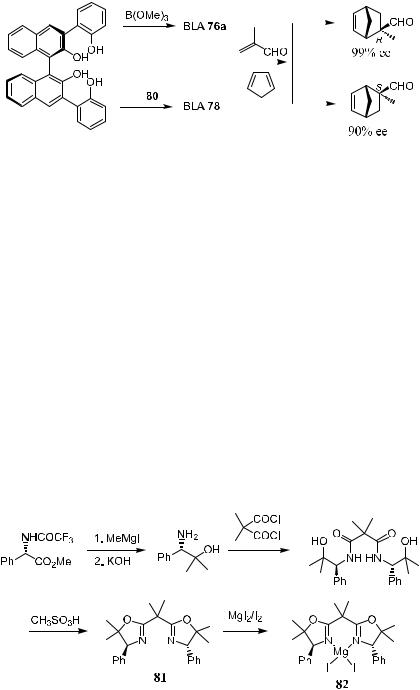
Yamamoto and co-workers found that BLA 76a is one of the best catalysts for the enantioselective and exo-selective cycloaddition of a-substituted a,b- enals to highly reactive dienes such as cyclopentadiene. The results in the presence of (R)-76a are summarized in Table 5±2. The major enantiomer has, in several cases, been demonstrated to have the predicted absolute con®guration.
Entries 6±8 show that with BLA 76, the corresponding reaction with a- substituted a,b-enals such as acrolein and crotonaldehyde exhibits low enantioselectivity and/or reactivity. However, the enantioselectivity can be raised from 40% to 90% ee by using (R)-76b instead of (R)-76a (Entries 6 and 7).
A more practical BLA has been designed to improve the scope of dienophiles in reactions with less reactive dienes. 3,5-Bis(tri¯uromethyl)- phenylboronic acid (80) was chosen as the Lewis acidic metal component in the new BLAs 77±79. These new BLAs show higher catalytic activity in enantioselective cycloaddition of both a-substituted and a-unsubstituted a,b-enal with various dienes.
The results suggest that BLA 77 is the best catalyst. For example, the reaction of (E )-methyl acrolein with cyclopentadiene catalyzed by 77 (5 mol%) gives the adduct at 96% yield with 99% ee [(S )-con®guration].
Studies also show that by using catalyst 76a or 78 derived from one single chiral tetraol with the same absolute con®guration, both enantiomers of the DielsAlder reaction product can be obtained. For example, reaction of 2-methyl-
5.4 CHIRAL LEWIS ACID CATALYSTS |
287 |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 5±24
acrolein with cyclopentadiene catalyzed by 76a yields the product with an (R)- con®guration, while reaction of 2-methylacrolein with cyclopentadiene catalyzed by 78 (5 mol%) yields the adduct with an (S)-con®guration (Scheme 5±24).
5.4.6Bis(Oxazoline) Catalysts
C2-symmetric bis(oxazoline) and salen±metal complexes have recently been shown to be very e¨ective ligands for iron(III),28 magnesium(II),29 copper(II),30 and chromium(III)31 complex-catalyzed reactions.
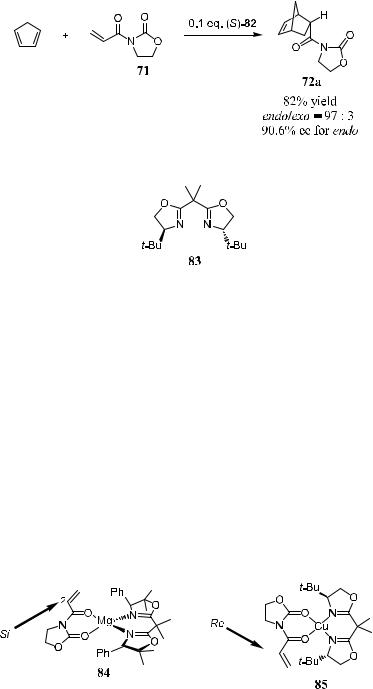
Corey and Ishihara29 report the synthesis of a new bis(oxazoline). This catalyst e¨ects Diels-Alder reaction via a tetracoordinated metal complex. Ligand (S)-81 is synthesized from (S)-phenylglycine, as depicted in Scheme 5±25. Treatment of 81 with MgI2 I2 gives a dark solution of complex 82, which can be utilized as a Diels-Alder reaction catalyst. Thus, reaction of cyclopentadiene with 71 in the presence of 82 yields product 72a with an enantiomeric ratio of over 20:1 (Scheme 5±26).
When a copper complex of 83 is used as the catalyst, the reaction can also proceed with excellent selectivity (98% de and >98% ee for the endo-isomer).30 Note that 83 is similar to 81, but in this compound the two phenyl groups are replaced by two t-butyl groups.
Scheme 5±25. Preparation of bis(oxazoline) magnesium(II) diiodide.
288 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
Scheme 5±26
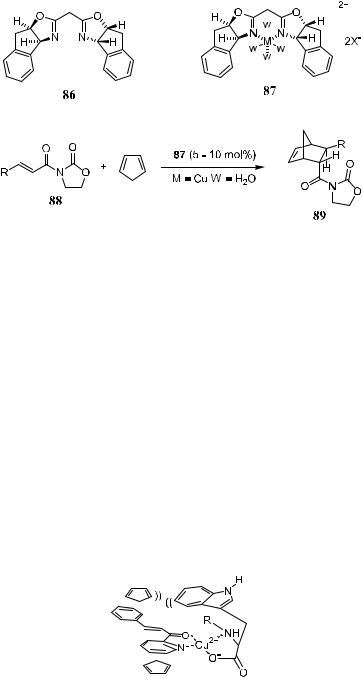
The explanation for the topicity of the two complexes that lead to opposite stereo-outcomes is presented in Figure 5±6. Even though they are similar C2 symmetric ligands, they coordinate with metals in di¨erent tetravalent geometry, which then follow di¨erent pathways to the products. The tetrahedral magnesium complex 84 facilitates cycloaddition to the C2 Si-face because of rear phenyl blocking of the Re-face. In contrast, the square±planar copper complex 85 favors Re-addition due to its Si-face being blocked by a t-butyl group.
Ghosh et al.32 have demonstrated another bis(oxazoline) derivative chiral ligand 86 for asymmetric Diels-Alder reaction and obtained excellent results. Reaction of an equimolar mixture of chiral ligand 86 and Cu(ClO4)2 6H2O produces the aqua complex 87 (w being water molecule), which shows excellent catalytic power in asymmetric Diels-Alder reactions. As depicted in Scheme 5± 27, the reaction of 88 with cyclopentadiene gives product 89 with more than 80% yield, over 99:1 diastereoselectivity and up to 99% ee.
For a recent review of chiral bis(oxazoline)-mediated asymmetric syntheses, see Ghosh et al.33
Figure 5±6. Rationale for the di¨erent topicities of bis(oxazoline) complexes.
5.4 CHIRAL LEWIS ACID CATALYSTS |
289 |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 5±27
5.4.7 Amino Acid Salts as Lewis Acids for Asymmetric Diels-Alder Reactions
Besides the Lewis acid catalysts discussed above, chiral amino acid copper salts have also been used for inducing asymmetric Diels-Alder reactions. In most of the above reactions, aprotic organic solvents were used as the reaction media, and donor solvents such as water or alcohol were generally avoided. This is partially explained by the fact that these solvents exert detrimental e¨ects, such as coordinating with the catalytic Lewis acid and thus deactivating the catalyst or diminishing the asymmetric catalytic activity of the catalyst. Otto et al.34 report that asymmetric Diels-Alder reactions catalyzed by amino acid copper salts can proceed with higher enantioselectivity in the presence of water. They studied the asymmetric Diels-Alder reaction of 3-phenyl-1-(2-pyridyl)-2- propen-1-one with cyclopentadiene in the presence of a series of amino acid salts. The Diels-Alder adducts can normally be obtained at over 90% yield after 48 hours of reaction. Signi®cant enantioselectivity has been observed with a- amino acids bearing aromatic side groups. This may be due to the possible p- stacking e¨ect in the transition state, as shown in Figure 5±7.
Figure 5±7. Transition state of amino acid salt catalyzed Diels-Alder reaction. Reprinted with permission by Am. Chem. Soc., Ref. 34.

290 ASYMMETRIC DIELS-ALDER AND OTHER CYCLIZATION REACTIONS
TABLE 5±3. Solvent E¨ect of Amino Acid Salt-
Catalyzed Diels-Alder Reaction
Entry |
Solvent |
ee (%) |
|
|
|
1 |
Acetonitrile |
17 |
2 |
THF |
24 |
3 |
Ethanol |
39 |
4 |
Chloroform |
44 |
5 |
Water |
74 |
ee ˆ Enantiomeric excess; THF ˆ tetrahydrofuran.
Reprinted with permission by Am. Chem. Soc., Ref. 34.
Otto et al. studied asymmetric Diels-Alder reactions in the presence of the copper salts of glycine, l-valine, l-leucine, l-phenylalanine, l-tyrosine, l- tryptophan, and N-a-l-tryptophan (l-abrine). The copper salt of l-abrine gave the highest enantioselectivity. Table 5±3 compares the solvent e¨ect in this reaction, and clearly water is the best solvent among the solvent systems studied.
5.5HETERO DIELS-ALDER REACTIONS
5.5.1Oxo Diels-Alder Reactions
When aldehyde containing an electron-withdrawing group is employed, or when a Lewis acid promoter is present, CbO double bonds can readily undergo Diels-Alder±type reactions. This process is referred to as the oxo Diels-Alder reaction, and it has been explored by Danishefsky and DeNinno35 for the synthesis of a wide range of saccharide derivatives.
The oxo Diels-Alder reaction, or the hetero Diels-Alder reaction involving a carbonyl compound as the dienophile, constitutes a useful method for carbon skeleton construction. Much attention has focused on developing e¨ective new catalysts for hetero Diels-Alder reactions over the last few years.36 Mikami et al.37 have reported that by using isoprene as a diene component, Diels-Alder product can be obtained in extremely high enantiomeric excess. For example, the reaction of 1-methoxy-1,3-butadiene proceeds smoothly in the presence of BINOL±TiCl2, giving the cis-product in high ee (Scheme 5±28).
Scheme 5±28
