
- •T. J. Djankova, a. A. Burinskaja, s. A. Zakharenkov technology of finishing textile materials
- •1. Principal views of textile fibers
- •2. Preparation of cellulose materials for dyeing and printing
- •2.1. Bleaching of cotton textiles
- •2.2. Mercerization
- •3. Application of optical bleaches
- •3.1. Optical bleaching substances
- •3.2. Test on presence of an optical bleach
- •4. Dyeing
- •4.1. Technical classification of dyes
- •4.3. Mordant dyes
- •4.4. Acid metalline dyes
- •The abovementioned recipe and procedure of dyeing are standart and can be changed and specified according to type of the equipment and also kind of coloring material.
- •4.5. Direct dyes Direct dyes may be used for dyeing cotton and other cellulose fibers. Direct dyes simple in application, are suitable for dyeing on any equipment, well combined with each other.
- •4.6. Reactive dyes
- •4.6.1. Cellulose dyeing. Batch methods of dyeing
- •Table 4.1. Dyes Bath Composition and Dyeing Conditions
- •4.6.2. Continuous dyeing
- •4.7. Cationic dyes
- •Dyeing by fast-fixing dyes
- •Dyeing of newly-formed braid
- •4.8. Disperse dyes
- •4.9. Vat dyes
- •Indigo-molecular structure Vat Yellow-molecular structure
- •Dye. . . . . . . . . . . . . . . . . . . . . .3 % from weight of a fiber
- •4.10. Sulfur dyes
- •4.11. Azo dyes synthesized in the fiber
- •5. Printing
- •5.1. Reactive dyes printing
- •5.2. Pigments printing
- •5.3. Thermoprinting of fibrous materials
- •6. Final finishing
- •6.1. Giving to fabrics of properties of water pushing away
- •6. 2. Giving to textile cloths of oil- hidrofobization
- •6.3. Giving to fabrics of fireproof properties
- •6.4. Giving to fabrics of anti-shrinkage chemical properties, form-stable finishing
- •Application Rules
- •7. Dyeing from Emulsions
- •7.1 Auxiliaries solvents
- •7.2 Emulsifiers
- •7.3 Dyeing with water-soluble dyestuffs.
- •7.4. Basic dyeable synthetic fibers
- •7.5. Physic-chemical fundamentals of emulsion technique
- •Influence of the temperature on the stability of an emulsion
- •Influence of additives on the stability of an emulsion
- •The optical properties of a water/perchloroethylene emulsion
- •Vapour pressure of a water/perchloroethylene emulsion
- •7.6 Equipment for dyeing from organic solvents
- •8. Equipment for dyeing and finishing factories.
- •8.1. Machine for washing, bleaching and dyeing “colorado”
- •8.2. Мachine «petra» f. Biancalani For obtaining effects of “worked surface”
- •8.3. High temperature machine mcs comby jigger
- •8.4. Hydraulic drying cylinder machines “jigger jht” by exclusivas tepp s.A. (Spain)
- •8.5. Vertical high-temperature high-pressure yarn dyeing plant
- •8.6. Flow line for combined bleaching and dyeing of fabrics лкб-140
- •Specification
- •8.7. Rapidstretch
- •8.8. Technodye rapid system Main features.
- •8.9. Superflux ne
- •Finally
- •8.7. Rapidstretch 84
4.10. Sulfur dyes
Sulfur dyes are the most commonly used dyes manufactured for cotton in terms of volume. They are cheap, generally have good wash-fastness and are easy to apply. The dyes are absorbed by cotton from a bath containing sodium sulfide or sodium hydrosulfite and are made insoluble within the fiber by oxidation. During this process these dyes form complex larger molecules, which is the basis of their good wash-fastness. These dyes have well all round fastness except to chlorine. Due to the highly polluting nature of the dye-bath effluent, slowly sulfur dyes are being phased out. Sulfur dyes are primarily used for dark colors such as blacks, browns, and dark blues. The deep indigo blues of denim blue jeans are a product of sulfur dyes. Recent advances in dyeing technologies have allowed the substitution of toxic sulfide reducing agents. Glucose is now used and both low sulfide and zero sulfide products are available. Future developments in the field of reducing dye levels by means of electro-chemical processes are promising. This work is just in the research stage but is expected to come to industry very soon. This may eradicate the problems of polluting sulfides. Sulfur dyes are water insoluble. They have to be treated with a reducing agent (Na2S) and an alkali (NaOH or Na2CO3) at temperature of around 80oC where the dye breaks into small particles which then becomes water soluble and hence can be absorbed by the fabric. Heating and adding a substance like common salt facilitates the absorption. After this the fabric is removed from the dye solution and then taken for oxidation.

During the oxidation step the small particles of dye once more form the parent dye which is insoluble in water. This oxidation can be done in air or by using oxidizing agents like hydrogen peroxide or sodium bromate in a mildly acidic solution. Now as the dye has become water insoluble in fiber so it will not bleed in water when washed and will not stain other clothes. However the dye may have poor fastness to rubbing, that is the dye from the fiber may come out gradually if the fabric is rubbed against. Also the fastness to hypochlorite bleach is poor because hypochlorite breaks the color imparting group in the dye and hence the colored part becomes colorless. Sulfur dyes are very inexpensive and very important to the dyeing industry. Out of all the sulfur dyes perhaps 50% of production is of the sulfur black color as black is the most popular fabric color. Sulfur dyes do not have any pure red color in its shade range. A pink or lighter scarlet color is available.
4.11. Azo dyes synthesized in the fiber
Azo dyes form a large structural group of synthetic dyes. An azo dye has the general structure (Ar–N=N–Ar') and is produced by the reaction of an aryldiazonium salt with an aromatic amine or a phenol. Aryl diazonium salts are prepared by treating an acidic solution of an aryl amine with sodium nitrite:

general structure of an azo dye
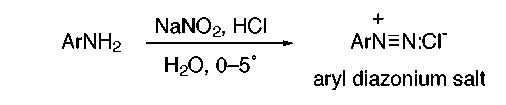
Aryl diazonium salts are stable for a reasonable period of time if kept in an aqueous solution at 0–5˚. They are relatively weak electrophiles but have sufficient reactivity to attack highly activated aromatic rings such as amines and phenols. The ensuing reaction, called “azo coupling”, results in molecules that have the –N=N– system linking two aromatic rings.

Azo dye compounds come in a broad range of colors, including yellows, oranges, reds, browns, and blues; it is the structure of the azo compound that determines the color that will be exhibited. The extensive conjugated π systems of azo dyes cause them to absorb at a certain wavelengths in the visible portion of the spectrum. When a compound absorbs a particular wavelength (color) from white light, the combination of the reflected wavelengths (colors) makes the compound appear colored. For example, if a compound absorbs in the visible region at 500 nm, which is green, the combination of all the other wavelengths which are reflected makes the compound appear red. In general, the more extensive the conjugated π system of a molecule, the longer the wavelength of visible light it will absorb, and the observed color will change accordingly.
White (least extensive π system) → yellow → orange → red → green → blue (most extensive)
In aromatic systems, another factor which increases the intensity and wavelength of the absorption is having a strong electron-donating group and a strong electron- withdrawing group para to one another on the aromatic ring. The electronically excited state produced upon absorption of light has dipolar character which is stabilized by these groups as shown below:

Since the excited state is stabilized relative to the ground state, the ground state absorbs light of lower energy (longer wavelength) than would be expected for an analogous molecule without the electron donating/withdrawing groups.
Here you can see synthesize the dye para red from para-ntiroaniline and ß-naphthol:

The mechanism for the reaction of aromatic amines or phenols with an aryl diazonium ion to form an azo compound is shown below:

Azo compound
An example of an ingrain dye is para red (“American flag red”), an ingrain dye for cotton. The fabric is soaked first in a solution of the coupling component ß-naphthol and then in a solution of diazatized p-nitroaniline.

Para red is one member of a large structural group of synthetic dyes called “azo dyes”. Synthesized by the coupling of aryl diazonium salts with various aromatic amines and phenols, azo dyes encompass a broad range of colors, including yellows, oranges, reds, browns, and blues. It is the structure of each particular dye that determines the color that will be exhibited. In general, the more the conjugated system of a molecule, the longer the wavelength of visible light it will absorb, and the observed color will.
The fabric is treated with the two components used to synthesize the dye. These precursor molecules are small enough to diffuse into the pores and spaces between the fibers in the fabric. They then react to form the dye, which is trapped inside or “in the grain” of the fibers because of the large size of the dye molecule.
Cotton

