
- •Preface to the Second Edition
- •Preface to the First Edition
- •Contents
- •Contributors
- •Compositional Analysis of Naphtha and Reformate
- •Basic Reactions of Reforming on Metal Catalysts
- •Chemistry of Bifunctional Metal–Acid Catalysis
- •Naphtha Hydrotreatment
- •Preparation of Reforming Catalysts
- •Optimization of Catalyst Pore Structure by Kinetics and Diffusion Analysis
- •Naphtha Reforming Over Zeolite-Hybrid-Type Catalysts
- •Deactivation by Coking
- •Catalyst Regeneration and Continuous Reforming Issues
- •Precious Metals Recovery from Spent Reforming Catalysts
- •Licensed Reforming Processes
- •Control Systems for Commercial Reformers
- •Modeling Catalytic Naphtha Reforming
- •Index
12
Precious Metals Recovery from Spent Reforming Catalysts
Horst Meyer and Matthias Grehl
W.C. Heraeus GmbH & Co.
Hanau, Germany
1INTRODUCTION
Given the importance of reforming as a refinery process, significant quantities of noble metal–containing catalysts are installed worldwide in the operating oil refineries. The value of these catalysts is quite significant, so it is without any doubt that at the end of a catalyst life cycle, when the oil refineries produce a spent catalyst, the precious metals recovery and other related aspects have to be handled with corresponding attention [1]. The modern petroleum-processing industry would not exist without precious metals catalysts, but the associated investment value brings with it special concerns. Most oil refineries already have installed a special care program incorporating guidelines beginning with the purchase of the fresh catalyst until the metals are recovered from the spent one. For each of the precious metals refiners, dealing with spent catalysts goes beyond showing the best possible recovery performance, but also acting as a consultant for the customer in respect to all precious metals–related technical procedures, including fine-metal and precious metals compounds handling, analytical procedures, sampling statistics, logistics, and book-keeping. This chapter explains some of the practical aspects to this high-value part of the naphthareforming operation. Certain perspectives were presented in the first edition of this book [2]. Further perspectives are added in this chapter.
459
460 |
Meyer and Grehl |
2MATERIALS HANDLING AT THE OIL REFINERY
When it comes to the point where an oil refinery has decided to perform a catalyst change-out, certain in-house procedures come into force. This is especially true for precious metals–containing catalysts, where a variety of important procedures have to be observed. Even though each oil refinery has its own procedures, a general chronological pattern of steps for execution for a catalyst change-out can be established.
1.Decision about the timing of a change-out
2.Choice of the manufacturer for the fresh catalyst (if applicable)
3.Choice of the precious metals refiner for the spent catalyst
4.Reactor unloading/skimming/packaging, etc.
5.Shipping the spent catalyst for further treatment and metals recovery
At the time of the basic decision for a change-out, it will be already known if the catalyst has to go through a regeneration process. Depending on the carbon and/ or hydrocarbon and/or halides concentration on the spent catalyst, regeneration might become necessary to provide a safe handling through all further steps, such as sampling and metals refining. A properly regenerated catalyst will guarantee an accurate sampling operation, resulting in representative samples that will show the actual precious metals content in the catalyst delivered. In addition, possible surcharges that are assessed in the case of excessive carbon, hydrocarbon, or moisture can be avoided. In case of an ex situ regeneration it will be very important to make contact with a corresponding regeneration company as early as possible. Planning ahead for such a step will avoid unnecessary delays which later on would affect the precious metal availability. When it comes to step 3 above, several important pieces of information have to be available:
Type of catalyst
Quantity
Catalyst properties
Expected availability
Kind of change-out
Regeneration in situ or ex situ (if applicable)
Availability of sample
Based on this information a precious metals refiner will be able to calculate the requested terms and conditions for the recovery.
At the time when a specialized company arrives to take care of the unloading of a reactor, certain aspects have to be observed to guarantee smooth handling later on. Depending on the kind of change-out, the catalyst to be unloaded can consist of various fractions, such as:
Precious Metals Recovery |
461 |
Inert balls, or support material
Dust and broken pellets or extrudates
Heel catalyst (material containing excessive carbon)
Clean catalyst
Contaminated catalyst
Decisions about classifying catalyst as clean or contaminated depend on the maximum limits of various contaminants given by the precious metals refiner. Unloaded material has to be dumped into suitable drums, flow bins, or big bags. All fractions have to be clearly and carefully labeled and stored in such a way that they can be identified later on. This is important to avoid confusion and intermixing of catalyst types, which might ultimately cause problems during precious metals refining.
Each drum should be labeled as follows:
Name and address of the oil refinery. Type of catalyst.
Gross and net weight.
Name and address of the precious metals refiner.
If applicable, drums have to be marked according to the appropriate transport and waste regulations.
As an additional safety feature, it is recommended to seal each drum before shipping. This ensures that through all the handling steps, catalyst (and precious metals) will not be lost without notice. The supplier of the spent catalyst has to employ very careful control to ensure that everything is properly handled and all relevant safety and transport regulations are obeyed when the catalyst is shipped for reclamation. The requirements are not just a formalism; they are necessary for the safety and protection of value during transport and metals recovery of the catalyst. When it comes to shipping, it will be the refinery’s decision either to use their own in-house logistics or to subcontract to the precious metals refiner. In the final analysis, the least price per truck and the possible convenience for the refinery will be the driving force in the decision.
At the end, the paperwork should consist of
Purchase order Invoice Packing list
Material declaration (tracking document/AVV number) Declaration if material is classified as hazardous or nonhazardous
If necessary, any declaration in accordance with transport regulations
In case of the material declaration, assistance should be available from the precious metals refiner.

462 |
Meyer and Grehl |
Figure 1 Business transactions in catalyst refining and catalyst life cycle.
Figure 1 gives an overview of possible business transactions during the catalyst life cycle. There are various options that can be chosen depending on the needs of the customer. The most common options are:
Fine metal will be delivered to a catalyst manufacturer.
Fine metal will be kept on an weight account.
Fine metal will be sold at actual rates.
3DESCRIPTION OF SPENT CATALYSTS
3.1.Form
Spent reforming and similar alumina-supported catalysts are typically spheres or extrudates with dimensions of 1mm or more. Typically spheres are used for moving-bed reactors and extrudates for fixed-bed reactors.
3.2.Support Material
The support material is made from high-surface-area alumina (mainly g-alumina) of various strengths and porosites. During the lifetime of the catalysts, overheating occurs at some areas in the reactor and phase transitions can take place converting alumina phases of high solubility to other alumina phases (g to a) with low solubility in caustic soda or sulfuric acid. Noble metal catalysts for other processes are also sent for metals recovery. As a result, a-alumina-, silica-, or zeolite-supported catalysts are also common [3,4]. Figure 2 presents typical forms of reforming catalysts in spherical, pellet, and extrudate shapes.

Precious Metals Recovery |
463 |
Figure 2 Catalysts—pellets, extrudates, and spheres.
3.3.Precious Metal Content and Promoters
The precious metals content including the rhenium content of fresh reforming catalysts varies from about 0.2% to more than 0.6%. Typical precious metals concentrations of fresh reforming catalysts are 0.3% or 0.375% of each. At least one of the precious metals is contained in any catalyst. Besides platinum and rhenium, some petrochemical processes use palladium, ruthenium, and iridium. Depending on the usage and the physical properties of a reforming catalyst, the precious metals concentration in the spent catalyst should be nearly the same as the corresponding fresh one.
Besides rhenium and iridium, other promoters such as tin, lead, arsenic, cadmium, and germanium are used. Depending on the usage of a given catalyst these metals are used, but they are not considered important enough for metals reclaiming. Catalysts are typically identified by the manufacturer’s code using a combination of letters and numbers like R-62, RG-482, and E-603.
3.4.Distribution of the Precious Metals
Starting with the unloading procedure from the reactor, the catalyst is usually separated into fractions such as fines, oversize, and whole catalyst. The precious
464 |
Meyer and Grehl |
metals content for the fines often shows variations of +50% compared to the catalyst, whereas the oversize frequently does not contain any precious metals. The percentage of the fines related to whole catalyst depends on how the catalyst was originally produced and its properties. Also, the use of the catalyst during the cycle in the reforming reactor can influence the percentage of fines and their precious metals content.
3.5.Impurities
Typical impurities on the catalyst are iron and other components of steel such as nickel and chromium. Metals typically contained in the crude oil, such as vanadium and manganese, may also be present, particularly if some upset has occurred. These elements and the additional catalyst promoters can influence the metals refining process. An example of an especially deleterious effect would be contamination of the aluminum solutions resulting from the metals refining process, which could prevent further usage of these solutions. Such a restriction would influence the economics of the refining process.
Other impurities, such as halides, carbon, and hydrocarbons, have to be observed as they could have a direct influence on the metals refining process. Halides usually result from the catalyst manufacturing process of the alumina carrier or the finished catalyst production. For the case of isomerization catalysts, chlorine is continuously added to the process resulting in much higher halide concentrations than for reforming catalysts, where chloride maintenance is of minor extent. Carbon and hydrocarbons result from the carbon or coke deposited during the reforming process in the reactor.
4WEIGHING, SIEVING, AND SAMPLING PROCEDURES
Because of the considerable value of the precious metals on the catalyst, sampling is the first very important step. Taking into consideration the accuracy of a precious metals analysis and the value involved, the lot size for sampling has to be limited to a certain quantity in order to obtain the most accurate measurement of noble metal value. It is without any question that each sampling step can be witnessed by either the customer or a representative.
During receipt of the material at the precious metals refiner’s warehouse an inspection of the containers is performed. Containers will be opened only after release by the customer or the customer’s representative. Gross, tare, and net weight of each individual drum, big bag, or container is determined during the sampling operations by means of a calibrated scale. Simultaneously, the customer’s packing list is checked to ensure that the delivery is in compliance
Precious Metals Recovery |
465 |
with the actual material shipped. Any differences or other issues, such as damaged containers, are reported to the production management and customer service department immediately for appropriate action.
For the sampling of spent catalysts, specially designed equipment for screening and sampling is necessary. The objective of the screening and sampling operation is to obtain representative samples of the spent whole catalysts and other precious metals–containing fractions, e.g., the fines fraction. The sampling method will be explained using the Hanau sampling equipment as an example.
After dumping the catalyst into a movable container, the container is placed on top of a sieving device where the catalyst is uniformly fed to a vibrating screener. A continuous separation process now takes place, dividing the material into four fractions, namely, fines, pellets (middle fraction), magnetics, and oversize. In two steps a cut is taken for the pellets and a first intermediate sample of approximately 0.5% is obtained. The mesh width of the sieves is selected according to the pellet shape and size to ensure optimal separation from oversize. Fines of each lot are usually accumulated to form a fines lot. Such a lot will be kept separate from the pellet lots. Figure 3 presents equipment used in screening and sampling of catalysts.
At the end of the sampling operation, a second intermediate sample (reduced in size) is deived from the first intermediate sample. This sample is divided into two portions of approximately 3kg each. One portion remains in an “as is” state whereas the other portion is ground to a particle size less than 200mm. Each of these 3-kg portions is then split by a rotary divider into eight samples. A representative 3-kg sample is also taken from the fines, which are then further ground and similarly split into eight samples. These representative samples of ground pellets, original pellets, original as-is pellets, and fines can then be distributed to the analytical laboratories of the refiner, the customer, and/ or an independent representative assigned by the customer. Such samples are usually sealed by both parties using a seal pad that will not allow an uncontrolled opening of the samples. For all necessary sampling steps, such as grinding and dividing, it is important to ensure that all the equipment involved is of sufficient quality to the high value of the catalyst and the required precision. Figure 4 shows a schematic diagram of the screening and sampling device.
5ASSAYS OF PRECIOUS METALS AND RHENIUM
5.1.Sample Handling and Analysis
Because of the statistics and inhomogeneity of the as-is material, ground material has to be used for the precious metals analysis. During handling of the samples humidity could be absorbed or desorbed resulting in a change of weight. Therefore, a reproducible mass is needed as a basis for the calculation of the

466 |
Meyer and Grehl |
Figure 3 Screening and sampling equipment.

Precious Metals Recovery |
467 |
Figure 4 Schematic diagram—screening and sampling device.
precious metals and rhenium contents, which is the ignited material. All the precious metals assays in the Heraeus laboratories and the customers’ laboratories are related to the ignited material. The loss on ignition (LOI) is determined gravimetrically using an electric furnace and platinum crucibles. The LOI for the settlement weight is determined for the as-is sample.
Without going into actual analytical details, the steps in the analyses of the precious metals and rhenium are as follows. The alumina support material will be digested in the analytical lab. Traces of precious metals and rhenium that are dissolved will be precipitated. After separation of the precious metals–free solution, the residue from the digestion step, the precipitate, and any nondissolved support material will be totally dissolved in several steps.
Precious metals and rhenium are determined by the ICP method using an internal standard and suitable dilutions. Usually three assays from each lot are executed. For more accuracy, a test sample with known content (which has been assayed by gravimetric methods) is determined in parallel to the actual samples. The procedure is a complete analogue from the digestion of the support until the final ICP determination. The results should not deviate from the theoretical content by more than +0.002%.
468 |
Meyer and Grehl |
Sampling procedure, weight per sample, quantity of samples, the corresponding analytical procedure for the loss on ignition and insolubles and the final evaluation of the analytical results will be provided by the precious metals refiner together with a refining offer or quotation. Regarding the precious metals, carbon, hydrocarbons, and other contractual fixed elements or compounds, analytical methods depend on the equipment of the laboratory involved and should be specified.
5.2.Accuracy, Splitting Limits, and Umpires
The results of the analyses are exchanged by correspondence with the customer. Based on a contractual agreement, it is typically agreed that the metal contents found by both parties’ laboratories should correspond within a specified splitting limit. If the results are in between the splitting limits, the arithmetic mean of the exchanged analysis will be the agreed final content for the settlement calculation. Typical splitting limits are between 0.5% and 3% (relative) depending on the precious metal involved.
However, in the event that the results are further apart than the splitting limits, a repeat analysis may be performed as specified in the contract with the customer. If even the repetition is outside the splitting limits, a technical consultation between the responsible analytical person of both parties should take place to resolve the differences. In case the differences are still not resolved, a set of sealed samples will be submitted to an umpire laboratory, as outlined in the contractual agreement.
The accuracy of the sampling and analytical procedures is very important. As an example, we consider an order of 100 metric tons of reforming catalyst. This order contains 350kg platinum with a total value of US $8 million. A mistake of 0.5% relative is equal to US $40,000. Such a high value of the noble metals content is one of the reasons that the sampling lot is small.
6TREATMENT ROUTES
6.1.Removal of Halides, Hydrocarbons, and Carbon
Depending on the concentration of carbon and/or hydrocarbons and/or halides and the process involved, the catalyst typically must go through a pretreatment. In most cases, such a treatment is executed by specialized companies offering their services to either oil refineries or precious metals refiners. Heraeus provides it own in-house equipment for carbon and hydrocarbon burnoff.
Precious Metals Recovery |
469 |
6.2.Acidic and Alkaline Leach Processes
To extract the precious metals and rhenium, only the catalysts with high surface alumina phases can be treated using the wet chemical route. The alumina support has to be dissolved leaving the platinum, palladium, or iridium as a residue. Alternatively, the metals could be dissolved off the support. However, due to the physical characteristics of the porous support material, precious metals and rhenium are somewhat strongly fixed to the support, so that removal from the alumina is not possible at a satisfying percentage of recovery. There have been processes to remove the platinum by solid-state chlorination or HCl leaching, but these methods did not progress beyond laboratory or pilot scale [5]. Also, the direct reaction of the ground catalyst with concentrated sulfuric acid had no economical success. A continuous autoclave process for the dissolution of alumina has not been used for the treatment of spent reforming catalysts [4].
For spent catalysts with high surface area alumina and reasonable contents of insolubles and carbon, hydrometallurgical approaches are most commonly employed. Alumina support is dissolved with either sodium hydroxide or sulfuric acid [4,6]. If adequate precautions are taken, platinum as a noble metal is not dissolved in sodium hydroxide or in sulfuric acid. Alumina dissolution takes place as shown in the following two reactions:
Alkaline leach process:
Al2O3 þ 2NaOH þ 3H2O ! 2Na AlðOHÞ4 |
ð1Þ |
Sulfuric acid leach process: |
|
Al2O3 þ 3H2SO4 ! Al2ðSO4Þ3 þ 3H2O |
ð2Þ |
After the leaching process, rhenium is in the aqueous phase and the platinum, palladium, or iridium is in the solid residue. The choice of either the alkaline or sulfuric acid leach process depends on the infrastructure to dispose of sidestreams, the equipment, and past experiences in the refiner’s environment.
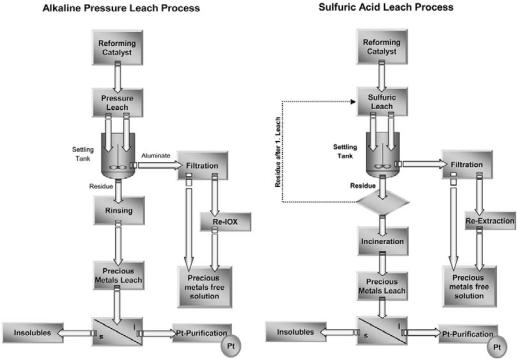
Both processes are operated at elevated temperatures. The alkaline process is also operated at elevated pressure during the first leach step. It is easily understood that elevated temperatures and pressures improve the process in regard to the dissolution time and quantity of nondissolved material. At Heraeus the alkaline pressure leach is used in Hanau, Germany, whereas sulfuric acid leach is used in Santa Fe Springs, California as shown in Table 1 and Figure 5.
6.3.Utilization of Leach Products
Aluminum sulfate and sodium aluminate are used in wastewater purification processes. Industrial customers purchase this material for their wastewater treatment plants. Having these industrial outlets is highly desirable because such
470 |
|
Meyer and Grehl |
Table 1 Alkaline and Sulfuric Leach Conditions |
|
|
|
|
|
|
Heraeus sulfuric acid leach |
Heraeus alkaline pressure leach |
|
|
|
Loading |
0.75–0.9 metric ton spent catalyst |
1 metric ton spent catalyst |
Leach chemical |
H2SO4 |
NaOH 50%, 45% |
Temperature |
,1008C |
180–2208C |
Pressure |
1bar |
8–24bar |
|
|
|
usage contributes to the economics of the overall recovery process. Three factors are important for the subsequent sale and use of aluminum solutions. These are impurities outside the specification levels, the availability of competing replacement chemicals like FeCl3, and logistic costs for transporting the solutions.
6.4.Smelting
Smelting of the spent catalyst to a slag and a metallic phase is another option. This approach is typically used if the carrier material is nonsoluble. In case of rhenium-containing catalysts, rhenium goes into the slag phase and will be lost
Figure 5 Schematic diagram—alkaline and sulfuric leach process.
Precious Metals Recovery |
471 |
for recovery. The recovery yield for iridium would be low compared to the results achieved utilizing the wet chemical route.
7PRECIOUS METALS SEPARATION AND PURIFICATION
7.1.Dissolution of Leach Residue
To recover platinum, the leach residue is dissolved, in batch reactors. This is typically done with aqua regia or hydrochloric acid and chlorine [reaction (3)] or other hydrometallurgical treatments [7]. For platinum purification, solvent extraction or classical precipitation and redissolution [reaction (4)] as well as ionexchange methods can be applied.
7.2.Purification Process
At Heraeus, the hexachloroplatinate salt is dissolved, followed by reprecipitation. This results in a very good separation from all potential impurities [8].
7.3.Platinum Metal Production
The conversion of platinum salt to platinum metal can be done by wet chemical reduction, by pyrolytic decomposition [reaction (5)] of the ammonium hexachloroplatinate, or by electrowinning processes [reaction (6)]. At Heraeus Hanau, the electrowinning process is the most important route for metallic platinum (Fig. 6). The electrolysis is operating with a daily capacity of 80kg. The platinum is deposited as fine black powder on water-cooled titanium anodes. The cathodic chambers are separated by a membrane from the anode chambers. The electrolysis is carried out continuously for 22h, and then the harvesting process takes place. This is followed by filtration, washing, drying, and incineration to the final sponge form. The sponge is quality controlled by analytical methods to guarantee catalytic grade purity [8]. Common commercial and ASTM grades of platinum are 99.95% and 99.99%. The following set of reactions shows the sequence of platinum conversion from salt to metal.
Platinum dissolution:
Pt þ 2HCl þ 2Cl2 ! H2PtCl6 |
ð3Þ |
Platinum salt precipitation: |
|
H2PtCl6 þ 2KCl ! K2PtCl6 þ 2HCl |
ð4Þ |
Platinum salt incineration: |
|
ðNH4Þ2PtCl6 ! Pt0 þ 2NH4Cl þ 2Cl2 |
ð5Þ |

472 |
Meyer and Grehl |
Figure 6 Platinum electrowinning process.
Platinum electrowinning: |
|
H2PtCl6 þ 4e ! Pt0 þ 2HCl þ 4Cl |
ð6Þ |
7.4.Recovery Schemes for Palladium and Iridium
In the case of palladium-containing spent catalysts, the palladium is also precipitated as the hexachloropalladium salt. The palladium purification is done by the common ammonia purification process. At the end of the purification process, the yellow salt is reduced and then dried and treated at higher temperatures to obtain the final palladium sponge.
Precious Metals Recovery |
473 |
In the case of platinumand iridium-containing catalysts, the alkaline leach residue is dissolved in a multistep operation. After the HCl/chlorine leach a second step is needed to dissolve the remaining alumina. Then the residue is leached again with HCl/chlorine. Solvent extraction is used for both platinumand iridium-containing mother liquors to separate the platinum and the iridium. The costs for this process, the cycle time, and especially the yield of the iridium depends mainly on the insolubles of the spent catalysts, the iron content of the spent catalyst, and the iridium loss into the aluminate solution. Iridium purification is done by hydrometallurgical method including solvent extraction, ion exchange, and electrolysis.
8RHENIUM SEPARATION AND PURIFICATION
8.1.Solvent Extraction or Ion Exchange
Various methods can be used to separate the rhenium from the aluminum sulfate or sodium aluminate solutions. Solvent extraction and ion exchange are the most common. At Heraeus both methods are applied. Heraeus favors the anionexchange method. The hot sodium aluminate is passed through ion-exchange columns, so that the perrhenate is fixed and chloride is released into solution. To release the perrhenate, the column has to be treated with sodium hydroxide, hydrochloric acid, and then iron chloride solution.
8.2.Purification of Ammonium Perrhenate and Perrhenic Acid
To obtain ammonium perrhenate, the perrhenic acid is converted to ammonium perrhenate, which is crystallized as shown in reactions (7) and (8). The ammonium perrhenate is then purified by recrystallization. Further purification is obtained by passing the ammonium perrhenate solution over a cation-exchange column as shown in reaction (9). The catalyst grade ammonium perrhenate is obtained by precipitation with ammonium hydroxide. After drying and analytical control the ammonium perrhenate is ready for packaging. Common catalyst grade ammonium perrhenate contains 69.4% Re according to the customer’s or refiner’s specification. Rhenium contained in more dilute mother liquors of precipitation and recrystallization processes is precipitated as potassium perrhenate, since this is much less soluble.
Ammonium perrhenate precipitation and perrhenic acid:
HReO4 |
þ NH4Cl ! NH4ReO4 þ HCl |
ð7Þ |
HReO4 |
þ NH4OH ! NH4ReO4 þ H2O |
ð8Þ |
474 |
Meyer and Grehl |
Ion-exchange purification to perrhenic acid:
NH4ReO4 þ Res-H ! HReO4 þ Res-NH4 |
ð9Þ |
9COMMERCIAL ASPECTS
In order to meet customers’ expectations and to cover their needs on a worldwide basis, Heraeus operates two refineries for spent precious metals catalysts (Hanau, Germany and Santa Fe Springs, California), where a leading share of the available market is treated. We estimate that about 10,000–15,000 metric tons reforming and other refining catalysts containing Pt, Pt/Re, Pt/Ir, and Pd are available worldwide for metals reclamation per year. Taking into consideration a typical value of approximately US $70 per kilogram of spent catalyst (based on average precious metals prices, May 2001), the total worldwide value for precious metals recovery is approximately US $0.7–1.0 billion.
The high value per kilogram for spent catalyst must always be kept in mind, particularly when evaluating treatment and possible pretreatment charges (like carbon burnoff or hydrocarbon stripping). Losses must be minimized. Costs increase considerably in cases when a metal lease is needed. Therefore, it is very important to have all technical parameters of the spent catalyst in hand prior to a pickup. This will enable the oil refinery and precious metals refiner to work on a fixed schedule, thus reducing time for a given metals lease. The resulting advantages will be to avoid unexpected additional costs and to reduce the overall costs of using these noble metal catalysts in reforming.
REFERENCES
1.Gallmeier, J. The recovery of platinum and rhenium from petroleum catalyst. In
Proceedings of a Seminar at the International Precious Metals Institute, Las Vegas, Nevada, 1997.
2.Rosso, J.P.; El Guindy, M.I. Recovery of Pt and Re from Spent Reforming Catalysts. In Catalytic Naphtha Reforming: Science and Technology; Antos, G. J., Aitani, A. M., Parera, J. M., Eds.; Marcel Dekker: New York, 1995; p. 395.
3.Feige, R.; Winkhaus, G. Aluminumoxid—Rohstoff fu¨r Ingenieurkeramik. Metall, 1986, 40, 598.
4.Hudson, L.K.; Misra, C.; Wefers, K. Aluminum oxide. In Ullmann’s Encyclopedia of Industrial Chemistry, A1, 1985, 557.
5.Thomas, M.; Grosbois, J. Verfahren zur Rueckgewinnung von Platin und Iridium aus edelmetallhaltigen Katalysatoren, Deutsches Patentamt No. DT 2454647,1976.
Precious Metals Recovery |
475 |
6.Hoestra, J.; Michalco, E. Recovery of Platinum from Deactivated Catalyst Composites. US Patent No. 2950965, 1960.
7.Meyer, H.; Grehl, M.; Stettner, M. Verfahren zum Lo¨sen von Edelmetallen aus edelmetallhaltigen Scheidgu¨tern. Deutsches Patentamt No. 19928029, 1999.
8.Meyer, H.; Grehl, M. Paper presented at the Dechema European Workshop on Spent Catalysts: Recycling and Disposal. Frankfurt/Main, Germany, 1999.
