
Metal-Catalysed Reactions of Hydrocarbons / 08-Hydrogenation of Alkenes and Related Processes
.pdf
HYDROGENATION OF ALKADIENES AND POLY-ENES |
387 |
Figure 8.8. Adsorbed states of isoprene (2-methyl-1,3-butadiene) as alternative π3 σ structures and their half-hydrogenated states, leading to the three isomeric 2-methyl butenes.115,117
Thus by matching observed distributions to those calculated by varying f , the value of this parameter can be estimated; it varies from about +0.24 to −0.75. Illustrative examples of calculated distributions are given in Table 8.12. A refinement of the procedure to allow different reactivities of the two π3 intermediates has only a marginal effect. The source of the effect of increasing electron density in proportion to the number of methyl substituents has been confirmed by quantum mechanical calculations,115 from which it must be concluded that when f is positive the hydrogen atom must be δ+, and when it is negative it must be δ−. We therefore have a potentially useful method for identifying the polarity of the H––M bond involved in the reaction, and hence perhaps for studying metal-support interactions.
TOFs for palladium black and various supported palladium catalysts vary enormously, probably due to differences in surface cleanliness rather than particle size, and values of f lie between +0.225 (Pd/CaCO3) and −0.015 (Pd/SiO2): they were mainly positive.115 With increasing amounts of gold or silver added to Pd/SiO2, they became more positive: values of Stot were greater than 96%, and values of TOF (based on surface Pd atoms titrated by CO) were larger (markedly so in some cases) than for Pd/SiO2.
The analysis has been extended to cover many of the published product selectivities found when hydrogenating isoprene on palladium catalysts.117 When

388 |
CHAPTER 8 |
f is less than −0.5, the 3,1-isomer is not formed, and when it is −1 the 2,2-isomer is the only possible product: such results are sometimes encountered. A particularly interesting set of results was obtained by selective poisoning of Pd/C with Ph3M (M = N, P, As, Sb, Bi):113 toxicity increased and values of f decreased with rise in atomic number of M, and it was thought that increasing electron donation counteracted the positive charge on the hydrogen atoms, so that f had to become more negative. Other systems analysed included one where marked solvent effects were revealed. In one extensive study of the effect of adding Group 14 elements (and Sb) to Pd/Al2O3, full product analyses were very unfortunately not recorded:112 constant values of TOF and of activation energy were (55 kJ mol−1) were observed, and Stot was increased, especially by lead. Changes with time-on-stream have been followed with eggshell Pd/δ-Al2O3 catalysts of various dispersions: conversion decreased and Stot increased: as observed elsewhere, faster rates were found with catalysts of lower dispersion.110
Platinum catalysts have given product distributions that were not consistent with the mechanism that seems to apply to palladium.118,119 Isoprene hydrogenation has also been performed on Pt(111);50 at 423 K, Stot was very low (37%). Raney nickel has also been used.120
The method of analysis described above can also be used on the homogeneously-catalysed reaction: a number of organometallic complexes have given product mixes that are understandable on the basis of π4 or π3σ intermediates.117
8.4.3. Cycloalkadienes2,44
Cyclopentadiene has received remarkably little attention:44 this is strange, because the molecule is planar and rigid, and should be invaluable for mapping the geometry of adsorption sites. On various copper catalysts, it is hydrogenated with 100% selectivity to cyclopentene with an activation energy of 54 kJ mol−1:121 its chemisorption is however weak, because the rate equation is
r = k PH PC L |
(8.3) |
where PC L indicates a dependence on cyclopentadiene pressure described by the Langmuir adsorption equation.
Reactions of cyclohexadienes will be considered in Chapter 12 as intermediates in the dehydrogenation of C6 cycles to benzene. There is no information on cycloheptadienes.
1, 3-Cyclo-octadiene is a conjugated diene, so that selective reduction to cyclo-octene should occur readily: it has also been only little studied. On Pd/pumice catalysts of various dispersions, TOFs decreased sharply as particle size fell in the
HYDROGENATION OF ALKADIENES AND POLY-ENES |
389 |
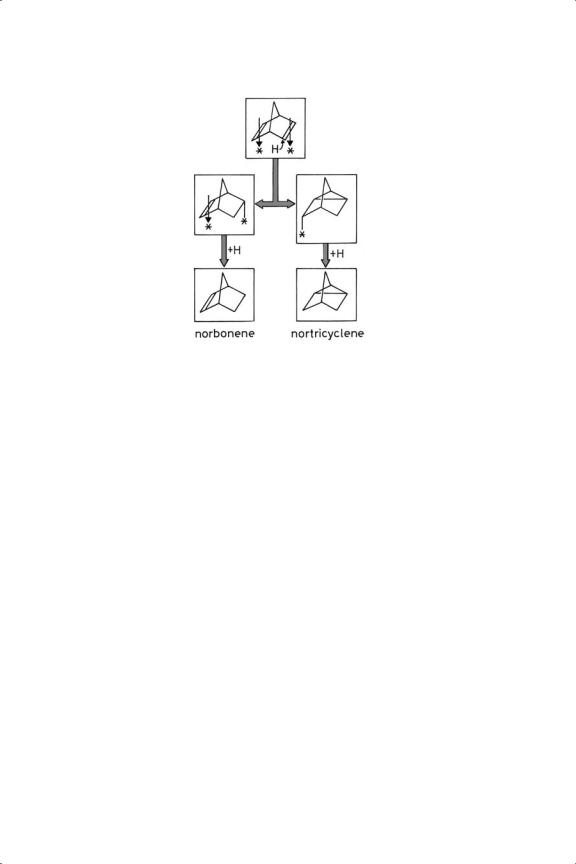
Figure 8.9. Addition of a hydrogen atom to norbornadiene (tricyclo[2.2.1]heptadiene) leading to norbornene and nortricyclene.
2 to 3 nm region (30% dispersion), maximum selectivity being found at about 50% dispersion.70,87 Sodium ion in pumice increased the rate of electron transfer to the metal, thus weakening the chemisorption bond.122 On Cu/TiO2 (Degussa P-25), preparation by slow deposition of hydroxide from an ammine solution by dilution with water gave a much more active catalyst than conventional impregnation.123 FeCu/SiO2 catalysts gave fully selective reduction of the diene.124
On an unspecified supported palladium catalyst at 323 K, 1,5-cyclo-octadiene isomerised to the 1,3-form at about the same rate as it was hydrogenated; Stot was about 90%, and some mathematical modelling was attempted.107 Other cyclic dienes have also been examined.125
Norbornadiene (bicyclo[2.2.1]octadiene) also has its two double bonds in a fixed geometry. On supported copper and gold catalysts above 353 K, and on Co/pumice and Pt/pumice above 333 K, nortricyclene and its isomer norbornene (Figure 8.9) were formed simultaneously, but not on Pt/MgO.126 Silver catalysts were inactive, as were all Group 11 metals for norbornene hydrogenation. A 1,3- dipolar addition of a hydrogen molecule was proposed, although the products are equally well accounted for by the mechanism shown in the Figure. Molecular addition may however be important in cases where the concentration of adsorbed hydrogen atoms is low, as with the Group 11 metals and Pt/TiO2 in the SMSI state. The activity of gold for this reaction passed without substantial comment.
390 |
CHAPTER 8 |
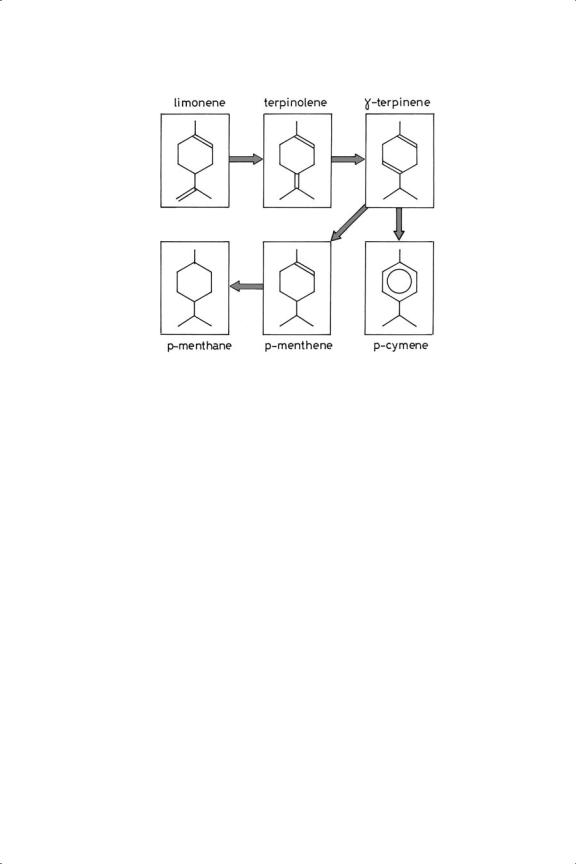
Figure 8.10. Hydrogenation of limonene (1-methyl-4-isopropenyl-cyclohexene).127
Extremely complex reaction paths are encountered in the hydrogenation of terpenes, by reason of the diversity of isomeric mono-enes and dienes that are possible: the presence of the C6 ring also allows dehydrogenation to an aromatic molecule to occur. A recent detailed study of the hydrogenation of limonene (1-methyl-4- isopropenyl-1-cyclohexene) illustrates well the complexity that can be found;127 it supplements many qualitative observations to be found in the literature.2 The principal pathway on Pd/C or Pd/Al2O3 at 273 and 323 K involved progressive double-bond migration as shown in Figure 8.10 to the 1,4-cyclohexadiene, which then (following the tendency of such molecules) suffered disproportionation to the mono-ene and aromatic molecule.
1, 5, 9-cyclododecatriene was hydrogenated progressively to a diene and the mono-ene on Pd/Al2O3 at 423 K, with maximum selectivities of respectively about 50 and 65%.128
REFERENCES
1.K.J. Klabunde, S.C. Davis, H. Hattori and Y. Tanaka, J. Catal. 54 (1978) 254; P. Herman, D. Simon, P. Sautet and B. Bigot, J. Catal. 167 (1997) 33.
2.P.N. Rylander, Catalytic Hydrogenation over Platinum Metals, Academic Press: New York (1967).
3.P.N. Rylander, Catalytic Hydrogenation in Organic Synthesis, Academic Press: New York (1979).
4.R. Kuhn and H. Fischer, Chem. Ber. 92 (1959) 1849; 93 (1960) 2285.
5.L. Guczi and A. S´ark´any in: Specialist Periodical Reports: Catalysis, Vol. 11 (J.J. Spivey and S.K. Agarwal, eds.), Roy. Soc. Chem. (1994), p. 318.

HYDROGENATION OF ALKADIENES AND POLY-ENES |
391 |
6.R.J. Farrauto and C.H. Bartolomew, Fundamentals of Industrial Catalytic Processes, Chapman and Hall: London (1997).
7.J.P. Boitiaux, J. Cosyns, M. Derrien and G. L´eger, Hydrocarbon Proc. (1985) 51.
8.J. Goetz, D.Yu. Murzin and R.A. Touroude, Ind. Eng. Chem. Res. 35 (1996) 703.
9.R.L. Moss in: Specialist Periodical Reports: Catalysis, Vol. 1 (C. Kemball and D.A. Dowden, eds.), Roy. Soc. Chem. (1977), p. 37; Vol. 4 (C. Kemball and D.A. Dowden, eds.), Roy. Soc. Chem. (1981), p. 36.
10.G. Webb in: Specialist Periodical Reports: Catalysis, Vol. 2 (C. Kemball and D.A. Dowden, eds.),
Roy. Soc. Chem. (1978), p. 145.
11.D.A. Dowden in: Specialist Periodical Reports: Catalysis, Vol. 2 (C. Kemball and D.A. Dowden, eds.), Roy. Soc. Chem. (1978), p. 1.
12.S. Siegel, Adv. Catal. 16 (1966) 123.
13.R.L. Augustine, Heterogeneous Catalysis for the Synthetic Chemist, Dekker: New York (1996).
14.P.B. Wells, in: Surface Chemistry and Catalysis (A.F. Carley, P.R. Davis, G.J. Hutchings and M.S. Spencer, eds.), Kluwer: Dordrecht (2003).
15.T. Ouchaib, J. Massardier and A. Renouprez, J. Catal. 119 (1989) 517.
16.J.P. Boitiaux, J. Cosyns and S. Vasudevan, Appl. Catal. 15 (1985) 317; J.P. Boitiaux, J. Cosyns and G. Martino in: Metal-Support and Metal-Additive Effects in Catalysis, (B. Imelik, C. Naccache, G. Coudurier, H. Praliaud, P. Meriaudeau, P. Gallezot, G.A. Martin and J.C. V´edrine, eds.), Studies in Surface Science and Catalysis, Elsevier: Amsterdam, 11 (1982) 355.
17.J. Barbier, E. Lamy-Pitara, P. Mar´ecot, J.P. Boitiaux, J. Cosyns and F. Verma, Adv. Catal. 37 (1990) 279.
18.G.C. Bond, Heterogeneous Catalysis – Principles and Applications, 2nd. edn., Oxford University Press: Oxford (1987).
19.B.I. Rosen, US Patent 4479902 (1984) to UOP Inc.
20.L.F. Albright, Chem. Eng. (1967) 249.
21.V.I. Savchenko and I.A. Makaryan, Platinum Metals Rev. 43 (1999) 74.
´
22. A. Molnar, A. S´ark´any and M. Varga, J. Molec. Catal. A: Chem. 173 (2001) 185. 23. G.C. Bond and P.B. Wells, Adv. Catal. 15 (1964) 92.
24. B.E. Bent, C.M. Mate, J.E. Crowell, B.E. Koel and G.A. Somorjai, J. Phys. Chem. 91 (1987) 3249.
25. E.F. Meyer and R.L. Burwell Jr., J. Am .Chem. Soc. 85 (1963) 2881.
26. J. Grant, R.B. Moyes, R.G. Oliver and P.B. Wells, J. Catal. 42 (1976) 213. 27. L.J. Shorthouse, S. Haq and R. Raval, Surf. Sci. 368 (1996) 296.
28. L. Crombie, P.A. Jenkins, D.A. Mitchard and J.C. Williams, Tetrahedron Lett. (1967) 4297. 29. G.C. Bond, PhD thesis, Birmingham University 1951.
30. G.C. Bond and J. Sheridan, Trans. Faraday Soc. 48 (1952) 658.
31. G.C. Bond and J. Sheridan, Trans. Faraday Soc. 48 (1952) 664. 32. R.S. Mann and A.M. Shah, Canad. J. Chem. 50 (1972) 1793. 33. R.S. Mann and D.E. Tiu, Canad. J. Chem. 46 (1968) 3249.
34. R.S. Mann and D.E. To, Canad. J. Chem. 46 (1968) 161.
35. R.S. Mann and A.M. Shah, Canad. J. Chem. 48 (1970) 3324. 36. Xing-Cai Guo and R.J. Madix, J. Catal. 155 (1995) 336.
37. R.G. Oliver and P.B. Wells, J.Catal. 47 (1977) 362.
38. A.J. Bates, Z.K. Leszczynski,´ J.J. Phillipson, P.B. Wells, and G.R. Wilson, J. Chem. Soc. (A) (1970) 2435.
39. G.V. Smith and R.L. Burwell Jr., J. Am. Chem. Soc. 84 (1962) 925.
40. R.G. Oliver, P.B. Wells and (in part) J. Grant, 5t h Internat. Congr. Catal. (J.W. Hightower, ed.), North Holland: Amsterdam 1 (1972) 659.
41. L. Crombie, P.A. Jenkins and D.A. Mitchard, J. Chem. Soc. Perkin Trans. I (1975) 1081.

392 |
CHAPTER 8 |
42.L. Crombie, P.A. Jenkins and J. Roblin, J. Chem. Soc. Perkin I (1975) 517.
43.W.R. Moore, J. Am. Chem. Soc. 84 (1962) 3788.
44.B.B. Corson in: Catalysis, (P.H. Emmett, ed.), Reinhold: New York, Vol. 5 (1957), p. 59.
45.N. Sheppard and C. de la Cruz, Adv. Catal. 41 (1996) 1.
46.J.C. Bertolini, A. Cassuto, Y. Jugnet, J. Massardier, B. Tardy and G. Tourillon, Surf. Sci. 349 (1996) 88.
47.J. Massardier, J.C. Bertolini, P. Ruiz and P. Delich`ere, J. Catal. 112 (1988) 21.
48.J. Oudar, S. Pinot and Y. Berthier, J. Catal. 107 (1987) 434.
49.C. Yoon, M.X. Yang and G.A. Somorjai, Catal. Lett. 46 (1997) 37.
50.C.-M. Pradier and Y. Berthier, J. Catal. 129 (1991) 356.
51.J. Oudar, S. Pinot, C.-M. Pradier and Y Berthier, J. Catal. 107 (1987) 445.
52.P. Hermann, J.M. Guigner, B. Tardy, Y. Jugnet, D. Simon and J.C. Bertolini, J. Catal. 163 (1996) 169.
53.S. Katano, H.S. Kato, M. Kawai and K. Domen, J. Phys. Chem. B 107 (2003) 3671.
54.R.B. Moyes, P.B. Wells, J. Grant and N.Y. Salman, Appl. Catal. A: Gen. 229 (2002) 251.
55.W.G. Young, R.L. Meier, J. Vinograd, J. Bottinger, L. Kaplan and S.L. Lindin, J. Am. Chem. Soc. 69 (1947) 2046.
56.K. Shimazu and H. Kita, J. Chem. Soc. Faraday Trans. I 81 (1985) 175.
57.K. Okamoto, K. Fukino, T. Imanak and S. Teranishi, J. Catal. 174 (1982) 173.
58.J.P. Boitiaux, J. Cosyns, and E. Robert, Appl. Catal. 35 (1987) 193; 32 (1987) 145.
59.J.J. Phillipson, P.B. Wells and G.R. Wilson, J. Chem. Soc. (A) (1969) 1351.
60.A. S´ark´any and Zs. R´evay, Appl. Catal. A: Gen. 243 (2003) 347; A. S´ark´any, Appl. Catal. A: Gen. 165 (1997) 87.
61.M. Okumura in ONRI Report No. 3. Aug. 1999, p. 54.
62.M. Okamura, T. Akita and M. Haruta, Catal. Today 74 (2002) 265.
63.B.J. Joice, J.J. Rooney, P.B. Wells and G.R. Wilson, Discuss. Faraday Soc. 41 (1966) 223.
64.P.B. Wells and (in part) A.J. Bates, J. Chem. Soc. (A) (1968) 3064.
65.G.C. Bond, G.Webb, P.B. Wells and J.M. Winterbottom, J. Chem. Soc. (1965) 3218.
66.M. George, R.B. Moyes, D. Ramarac and P.B. Wells, J. Catal. 52 (1978) 486.
67.A. S´ark´any, J. Catal. 180 (1998) 149; React. Kinet. Catal. Lett. 68 (1999) 153.
68.A. S´ark´any, Appl. Catal. A: Gen. 175 (1998) 245.
69.A. S´ark´any, Z. Schay, Gy. Stefler, L. Borko,´ J.W. Hightower and L. Guczi, Appl. Catal. A: Gen. 124 (1995) L181.
70.A.M. Venezia. A. Rossi, D. Duca, A. Martorana and G. Deganello, Appl. Catal A: Gen. 125 (1955) 113.
71.J.W. Hightower, B. Furlong, A. S´ark´any and L. Guczi, Proc. 10th. Internat. Congr. Catal. (L. Guczi, F. Solymosi and P. T´et´enyi, eds.), Akad´emiai Kiado:´ Budapest C (1993) 2305.
72.A.G. Burden, J. Grant, J. Martos, R.B. Moyes and P.B. Wells, Faraday Discuss. Chem. Soc. 72 (1981) 97.
73.P.B. Wells, J. Catal. 52 (1978) 498.
74.M. Primet, M. El Azhar and M. Guenin, Appl. Catal. 58 (1990) 241.
75.S.D. Jackson, G.D. McLellan, G. Webb, L. Conyers, M.B.T. Keegan, S. Mather, S. Simpson, P.B. Wells, D.A. Whan and R. Whyman, J. Catal. 162 (1966) 10.
76.D.A.G. Aranda and M. Schmal, J. Catal. 171 (1997) 398.
77.M. Schmal, D.A.G. Aranda, R.R. Soares, F.E. Noronha and A. Frydman, Catal. Today 57 (2000) 169.
78.A. S´ark´any, Z. Zsoldos, G. Steffler, J.W. Hightower and L. Guczi, J. Catal. 141 (1993) 566.
79.H. Miura, M. Terasaka, K. Oki and T. Matsuda, Proc. 10th Internat. Congr. Catal. (L. Guczi, F. Solymosi and P. T´et´enyi, eds.), Akad´emiai Kiado:´ Budapest C (1993) 2379.
80.M. Komiyama, K. Ohashi, Y. Morioka and J. Kobayashi, Bull. Chem. Soc. Japan 70 (1997) 1009.

HYDROGENATION OF ALKADIENES AND POLY-ENES |
393 |
81.V. de Gouveia, B. Bellamy, Y. Hadj Romdhane, A. Masson and M. Che, Z. Phys.: Atoms, Molecules and Clusters 12 (1989) 587.
82.A. Berthet, A.L Thomann, F.J. Cadete Santos Aires, M. Brun, C. Deranlot, J.C. Bertolini, J.P Rozenbaum, P. Brault and P. Andreazza, J. Catal. 190 (2000) 49.
83.R. Tardy, C. Noupa, C. Leclereq, J.C. Bertolini, A. Hoareau, M. Terilleux, J. F. Faure and G. Nihoul, J. Catal. 129 (1991) 1.
84.J.P. Boitiaux, J. Cosyns and S. Vasudevan, Appl. Catal. 6 (1983) 41; 15 (1985) 317.
85.J.C. Bertolini, P. Delichere, B.C. Khanra, J. Massardier, C. Noupa and B. Tardy, Catal. Lett. 6 (1990) 215.
86.J. Goetz, M.A. Volpe and R. Touroude, J. Catal. 164 (1996) 369.
87.G. Deganello, D. Duca, G. Fagherazzi and A. Benedetti, J. Catal. 150 (1994) 127.
88.P. Miegge, R.L. Rousset, B. Tardy, J. Massardier and J.C. Bertolini, J. Catal. 149 (1994) 404.
89.P.F. Carr and J.K.A. Clarke, J. Chem. Soc. A (1971) 985.
90.L.J. Shorthouse, Y. Jugnet and J.C. Bertolini, Catal. Today 70 (2001) 33.
91.L. Lianos, Y. Debauge, J. Massardier, Y. Jugnet and J.C. Bertolini, Catal. Lett. 44 (1997) 211.
92.A.C. Krauth, G.H. Berstein and E.E. Wolf, Catal. Lett. 45 (1997) 177.
93.K.S. Sim, L. Hilaire, F. Le Normand, R. Touroude, V. Paul-Boncour and A. Percheron-Guegan,
J.Chem. Soc. Faraday Trans. 87 (1991) 1453.
94.A. Bahia and J.M. Winterbottom, J. Chem. Tech. Biotech. 60 (1994) 305.
95.A. Bahia, I.R. Harris, C.E. King and J.M. Winterbottom, J. Chem. Tech. Biotech. 60 (1994) 347.
96.A. Bahia, I.R. Harris, C.E. King and J.M. Winterbottom, J. Chem. Tech. Biotech. 60 (1994) 337.
97.T. Komatsu, S. Hyodo and T. Yashima, J. Phys. Chem. B 101 (1997) 5565.
98.A.H. Weiss, S. LeViness, V. Nair, L. Guczi, A. S´ark´any and Z. Schay, Proc. 8t h Internat. Congr. Catal. Verlag Chemie: Weinheim 5 (1984) 591.
99.V. de Gouveia, B. Bellamy, A. Masson and M. Che in: Structure and Reactivity of Surfaces (C. Morterra, A. Zecchina and G. Costa, eds.), Studies in Surface Science and Catalysis’, Elsevier: Amsterdam, 48 (1989) 347.
100.A. S´ark´any, M. Zsoldos, J.W. Hightower and L. Guczi in: Science and Technology in Catalysis 1994, Kodanska: Prague (1994), p. 99.
101.S. Verdier, B. Didillon, S. Morin and D. Uzio, J. Catal. 218 (2003) 288.
102.P.B. Wells and G.R. Wilson, J. Chem. Soc. (A) (1970) 2242.
103.E.A. Sales, M.de J. Mendes and F. Bozon-Verduraz, J. Catal. 195 (2000) 96.
104.G.C. Bond and J.S. Rank, Proc. 3rd . Internat. Congr. Catal. (W.M.H. Sachtler, G.C.A. Schuit and
P.Zwietening, eds.), North Holland: Amsterdam 2 (1965) 1225.
105.R. Brayner, G. Vian, G.M da Cruz, F. Fi´evet-Vincent, F. Fi´evet and F. Bozon-Verduraz, Catal. Today 57 (2000) 187.
106.L. Horner and I. Grohmann, Liebigs Ann. 670 (1963) 1.
107.M. Di Serio, V. Balato, A. Dimiccoli, M. Mattucci, P. Iengo and E. Santacesaria, Catal. Today 66 (2001) 403.
108.N. Vasquez and R.J. Madix, J. Catal. 178 (1998) 234.
109.P.B. Wells and G.R. Wilson, Discuss. Faraday Soc. 41 (1966) 237.
110.J.-C. Chang and T.-C. Chou, Appl. Catal. A: Gen. 156 (1997) 193.
111.C. Lange, S. Storck, B. Tesche and W. F. Maier, J. Catal. 175 (1998) 280.
112.H.R. Aduriz, P. Bodnariuk, B.Coq and F. Figu´eras, J. Catal. 129 (1991) 47.
113.Y. Fujii and J. C. Bailar Jr., J. Catal. 52 (1978) 342.
114.P. Kripylo and D. Klose, Chem. Tech. (Leipzig) 29 (1977) 322.
115.G.C. Bond and A.F. Rawle, J. Molec. Catal. A: Chem. 109 (1996) 261.
116.J.-R. Chang, T.-B. Lin and C.-H. Cheng, Ind. Eng. Chem. Res. 36 (1997) 5096.
117.G.C. Bond, J. Molec. Catal. A: Chem. 118 (1997) 333.
118.R.L. Burwell Jr., Langmuir 2 (1986) 2.

394 |
CHAPTER 8 |
119.G.C. Bond, F. Garin and G. Maire, Appl. Catal.41 (1988) 313.
120.S. Sane, J.M. Bonnier, J.P. Damon and J. Masson, Appl. Catal. 9 (1984) 69.
121.G. Pajonk, M.B. Taghavi and S.J. Teichner, Bull. Soc. Chim. France (1975) 983.
122.L.F. Liotta, A.M. Venezia, A. Mortorana and G. Deganello, J. Catal. 171 (1997) 177.
123.F. Boccuzzi, A. Chiorino, M. Gargano and N. Ravasio, J. Catal. 165 (1997) 129, 140.
124.Y. Nitta, Y. Hiramatsu, Y. Okamoto and T. Imanaka, Proc 10th Internat. Congr. Catal. (L. Guczi,
F.Solymosi and P. T´et´enyi, eds.), Akad´emiai Kiado:´ Budapest C (1992) 2333.
125.I. Jardine and F.J. McQuillin, J. Chem. Soc. (C) (1966) 458.
126.V. Amir-Ibrahimi and J.J. Rooney, J. Molec. Catal. 67 (1991) 339.
127.R.J. Grau, P.D Zgolicz, C. Gutierrez and H.A. Taher, J. Molec. Catal. A: Chem. 148 (2000) 203.
128.F. Stuber,¨ M. Benaissa and H. Delmas, Catal. Today 24 (1995) 95.
129.B.K. Fourlong, J.W. Hightower, T.Y.L. Chan, A. S´ark´any and L. Guczi, Appl. Catal. 117 (1994) 41.
130.D.A. Buchanan and G. Webb, J. Chem. Soc. Faraday Trans. 71 (1975) 134.
131.A. S´ark´any, Z. Zsoldos, G. Steffler, J.W. Hightower and L. Guczi, J. Catal. 157 (1995) 179.
132.A. S´ark´any, Appl. Catal. A: Gen. 149 (1997) 207.
133.S.D. Jackson, J. Willis, G.J. Kelly, G.D. McLellan, G. Webb, S. Mather, R.B. Moyes, S. Simpson, P.B. Wells and R. Whyman, Phys. Chem. Chem. Phys. 1 (1999) 2573.
134.C.-Q. Liu, Y. Xu, S.-J. Liao and D.-R. Yu, Appl. Catal. A: Gen. 172 (1998) 23.
135.G.C. Bond, Catalysis by Metals, Academic Press: London (1962).
136.S.D. Jackson, M.B.T. Keegan, G.D. McLellan, P.A. Meheux, R.B. Moyes, G. Webb, P.B. Wells,
R.Whyman and J. Willis, in: Preparation of Catalysts V (G. Poncelet, P.A. Jacobs, G. Grange and B. Delmon, eds.) Elsevier: Amsterdam,( 1991), p. 135.
137.J.T. Wehrli, D.J. Thomas, M.S. Wainwright, D.L. Trimm and N.W. Cant, Proc. 10th . Internat. Congr. Catal. (L. Guczi, F. Solymosi and P. T´et´enyi, eds.), Akad´emiai Kiado:´ Budapest C (1993) 2289.
138.A. Borgna, B. Moraweck, J. Massardier and A.J. Renouprez, J. Catal. 128 (1991) 99.
139.Sun Hee Choi and Jae Sung Lee, J. Catal. 93 (2001) 176.
