
Neutron Scattering in Biology - Fitter Gutberlet and Katsaras
.pdf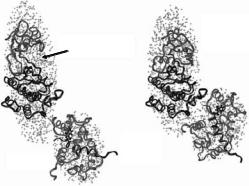
152 J.K. Krueger et al.
MLCK |
|
Plus AMPPNP and |
|
peptide substrate |
|
|
Catalytic Cleft |
|
|
|
MLCK-I
peptide
CaM
Fig. 8.7. Two ellipsoid models derived from the neutron scattering data for the 4Ca2+–CaM–MLCK complexes with (right, [115]) and without (left, [113]) substrates. The conserved portion of the inhibited kinase catalytic core [117] and the NMR structure of CaM complexed with MLCK-I peptide [15] are fit within the dimensions of the larger and the smaller ellipsoids, respectively. The upper and lower lobes of the catalytic core, with the catalytic cleft (labeled) between them, are represented as gray and black ribbon drawings. CaM is represented as a gray ribbon drawing, with its bound MLCK-I peptide in black, and a CPK representation of its hydrophobic Trp residue near the N-terminal end. This Trp residue is key to recognition and binding by the C-terminal CaM domain. This figure is adapted from [115]
phosphorylation sequence for myosin regulatory light chain) [115]. Comparison of the Rg values determined for the complex with bound substrates (31.6 ± 1.2 ˚A) to that without substrates present (34 ± 0.7 ˚A) indicated that there are significant structural di erences. As would be expected, Rg and P (r) analysis of the basic scattering functions determined for the CaM component was similar for the two complexes (18.1 ± 1.5 vs. 17.3 ± 0.4 ˚A). The Rg determined from the basic scattering function for the MLCK component decreases by almost 3 ˚A upon binding substrates indicating a compaction of MLCK that is also reflected in the P (r) analysis. This observed compaction of MLCK upon substrate binding is similar to that arising from the closure of the catalytic cleft in cAMP-dependent protein kinase upon binding pseudosubstrate. In addition, the distances between the centers-of-mass of the two components in each complex were determined from the basic scattering function cross-terms to be 57 ± 9 and 49 ± 10 ˚A.
A newer version of the MC integration modelling procedure, SASMODEL [82], was used to systematically test uniform-density two-ellipsoid models for the CaM–MLCK complexes against all of the scattering data. This twoellipsoid modelling exercise was ideal for this system because there was good evidence that both CaM and MLCK had compact globular structures. Figure 8.7 shows how the known high-resolution structure of CaM complexed with the 20 residue MLCK-I helical peptide [116] and the conserved

8 SANS from Biological Molecules |
153 |
catalytic core of the kinase (based on the cAMP-dependent protein kinase structure [117]) fit within the ellipsoid shapes derived from the scattering data. The empty spaces in the ellipsoid representing MLCK most likely are occupied by N-terminal and C-terminal sequence segments whose structures have not yet been determined. The center-of-mass separation between the two ellipsoids in the models are 57 ˚A for the minus substrate complex and 45 ˚A for the plus substrates complex. These values are consistent with those values determined from analysis of the basic scattering function of the cross-terms.
The models show that CaM binds to the kinase such that there must be a significant movement of the CaM-binding and autoinhibitory sequences away form the surface of the catalytic core. Upon binding substrates there is a movement of CaM approximately 12 ˚A closer to the catalytic cleft. Additionally, the models suggests that there is a reorientation of CaM with respect to the kinase that results in interactions between the N-terminal sequence of CaM and the kinase that were not observed in the complex without substrates.
The neutron scattering and contrast variation data presented a structural view which follows sequentially the conformational transitions in the CaMdependent activation of MLCK in solution. These studies provided important structural data that defined the mechanistic steps in the release of autoinhibition of MLCK by CaM, as well as subsequent substrate binding and activation.
8.4.3 Mechanism of the CaM-Activation Step: SAXS/SANS Studies of a (Deuterated) Mutant CAM
Neutron scattering and contrast variation experiments on the CaM-dependent activation of MLCK in solution have elucidated the sequential conformational transitions involved in the CaM-dependent activation mechanism of MLCK. Structural data determined from solution scattering experiments in combination with high resolution structural data on the individual components have defined the mechanistic steps responsible for the release of autoinhibition of MLCK by CaM, as well as subsequent substrate binding and activation. Additionally, a 2Ca2+ intermediate had been proposed based on spectroscopic studies [118,119] and this was further supported by small-angle X-ray scattering [120]. The purpose of such an intermediate could be to restrain the CaM from di using away in rapidly cycling functions such as muscle contraction and relaxation. Since the Ca2+ a nities of CaM are strongly a ected by its di erent target binding sequences, it has been further suggested that CaMbinding sequences in di erent enzymes may serve the purpose of “tuning” the calcium a nities of the Ca2+-binding sites so as to optimize for the formation of such intermediates when needed. CaM’s N-terminal lobe would possess the regulatory function, alternately binding and releasing the autoinhibitory sequence of MLCK in response to the Ca2+ signal.
The modeling program GA STRUC was used to generate low-resolution models for three complexes; 2Ca2+–CaM/MLCK, 4Ca2+–CaM/MLCK, and

154 J.K. Krueger et al.
Fig. 8.8. Results of shape restoration for 2Ca2+–CaM/MLCK SAXS data [115]. Three orthogonal views of consensus envelope from GA STRUCT (top). The conserved catalytic core for protein kinases in the open cleft conformation [117, 121] and collapsed CaM (2BBK [108]) structures have been docked by hand into the consensus envelope. The high-resolution structures resulting from the docking of the extended cPKA structure and collapsed CaM structure (2BBK) are shown in two views (the 2Ca2+–CaM/MLCK (grey) and 4Ca2+–CaM/MLCK (black) (bottom). The two complexes are overlaid such that the cPKA structures are coincident. The resulting view shows how far the CaM translocates away from the catalytic cleft of skMLCK when all four Ca2+ binding sites are occupied
4Ca2+–CaM/MLCK with bound substrate. These models were used in conjunction with high-resolution structures of the protein components to better understand the interactions between them (Fig. 8.8 [98]). In the case of the 2Ca2+–CaM/MLCK, the consensus envelope is consistent with CaM in a fully collapsed state with its two globular lobes in close contact with each other while the catalytic cleft of the kinase is open. The consensus envelope for the 4Ca2+–CaM/MLCK indicates that the collapsed CaM has swung further away from the open catalytic cleft of the MLCK compared to the 2Ca2+ complex, and further that substrate binding to this complex results in closure of the kinase catalytic cleft, in agreement with previous neutron scattering results. Most importantly, the GA STRUCT models indicate that activation
8 SANS from Biological Molecules |
155 |
of MLCK by CaM can only occur once CaM is fully translocated away from the catalytic cleft, which is presumably linked to full release of the pseudosubstrate/inhibitory sequence and this step is completed only when all four calcium binding sites are loaded.
All amino acid residues in CaM make up the two globular Ca2+-binding domains with the exception of residues 76–81 found in the central helix and residues 1–8 (A1DQLTEEQ8) at the N-terminus, hereinafter referred to as the N-terminal leader sequence. It has been shown that deletion of the N-terminal leader sequence results in a CaM mutant (DNCaM) capable of recognizing and binding to MLCK yet incapable of activating this kinase [122].
SANS contrast variation data on a specifically deuterated DNCaM mutant bound to MLCK has been collected. It is anticipated that analysis of this data will provide a more detailed atomic description of the binding events between CaM and MLCK prior to the kinase activation step, providing a molecular description of the regulatory mechanism for an archetypal calmodulin-mediated Ca2+ response in the cell. A high-resolution model of the DNCaM–MLCK complex has to be built from available atomic-resolution structures of CaM and the catalytic core of MLCK within the confines of the molecular envelope shapes, boundaries and relative dispositions. A docking procedure can then be used to develop “best fit” models of the complex similar to the procedure report recently by Tung et al., [123] that was used to develop a structural model of the catalytic subunit-regulatory subunit dimeric complex of the cAMP-dependent protein kinase (Fig. 8.9). The detailed “atomic” modeling presented in this article is an example of how the shape constraints provided by SANS can be combined with high-resolution crystal or NMR structure information and further constrained by other biophysical measurements. Higher detail models built from high-resolution crystal and NMR data of various substructures within the molecular dimensions determined from SAS and constrained by interresidue distances determined, for example, from chemical cross-linking and peptide mapping or FRET distances or mutagenesis data, is poised to become an important tool for an integrated structural approach to visualizing the protein:protein interactions that are essential for intracellular function. Approaches to modeling protein complexes with hybrid experimental data will become increasingly important as crystallographers continue to rapidly grow the structural data base with domain and subunit structures and we begin to turn our attention to understanding how these substructures function in the complex interactions within the cell.
8.5 Conclusions and Outlook
Neutron scattering with contrast variation can provide unique views of the interactions within molecular complexes involved in dynamic processes such as enzyme activation as well as in the highly regulated and coordinated interactions of complex systems such as muscle. The ability to selectively label

156 J.K. Krueger et al.
components in an assembly and extract information about their conformations within that assembly can be quite powerful. SAS techniques are applied in solution and hence can give insights into systems in which inherent flexibility may cause problems for crystallization. Importantly, the techniques can be applied to systems over a wide range of sizes, from 10 to 1000s of ˚A. Neutron scattering does su er the limitation that it requires access to large, expensive facilities of which there are a limited number. Thus, the techniques should only be applied when they can contribute unique information on an important problem. Understanding the molecular mechanisms underlying motility in biological systems and its regulation is one such problem. The complexity and the dynamic nature of motile function make it a very compelling system for study using neutrons.
As mentioned in the contribution by Harroun et al. in this volume, biology can be an educational outreach tool, that can connect with the public and policy makers in ways that many physics experiments cannot, particularly if they have some relevance to advances in medicine. This has had the effect that new instruments devoted to biological sciences such as the dedicated biological Advanced Neutron Di ractometer/Reflectometer (AND/R) at NIST are coming on line. In addition, a new 35 m SANS facility at ORNL [42] is being constructed as part of a Center for Structural and Molecular Biology (CSMB).
Finally, it may be worth re-emphasizing a point made initially in the context of SANS studies of synthetic polymers [124]: “The greatest limitation for SANS experimentalists is the securing of suitable samples. To take full advan-
B
A
Fig. 8.9. Best fit model for the R–C heterodimer of the cAMP-dependent protein kinase shows the dimer poised for dissociation. C (catalytic) subunit is shown as surface representation and the R (regulatory) subunit is shown as a cartoon representation of the backbone structure with some residues identified to be at interface, in ball-and-stick, labeled
8 SANS from Biological Molecules |
157 |
tage of the power of SANS, samples should be selectively deuterated in designated places.” Similarly, deuteration, partial or full, of biological molecules such as proteins, nucleic acids, lipids, sugars, is essential to exploit fully the techniques of neutron scattering and to highlight and analyze selected parts of macromolecular structures in situ. The commitment of a small fraction of the planned large investments in instrumentation to an in vivo labeling program will dramatically increase the overall impact and productivity of future research on biopolymers. The ILL in collaboration with European Molecular Biology Laboratory, has established a laboratory for the deuteration of biological molecules [125]. Similarly, as part of its strategy for the expansion of neutron scattering in the life sciences, the CSMB is planning a Deuterium Labeling Facility at ORNL. The provision of deuterated macromolecules will greatly enhance both the quality and quantity of experiments that can be done using neutron scattering, and in many cases will make feasible new and more sophisticated experiments than can presently be performed.
Acknowledgments
The work at Oak Ridge was supported by the Division of Materials Science, U.S. Department of Energy under contract DE-AC05-00OR22725 with the Oak Ridge National Laboratory, managed by UT-Battelle, LLC. The work at University of North Carolina Charlotte was supported by the National Science Foundation CAREER award MCB-0237676. The authors would like to thank D.M. Engelman (Yale University) who provided Fig. 8.3, C.-S. Tung who provided Fig. 8.9 and G. Zaccai for helpful advice in understanding the factors, which a ect the partial specific volumes of biological molecules. They also wish to acknowledge their many co-workers for permission to include data from their joint publications, particularly J.M. O’Reilly, V. Ramakrishnan, J. Trewhella, and W.T. Heller.
References
1.J. Chadwick, Nature 129, 312 (1932)
2.T.E. Mason, A.D. Taylor, Mat. Res. Soc. Bull. 24, 14 (1999)
3.P. Lindner, T. Zemb, Neutron, X-ray and Light Scattering (Elsevier Publishers, New York, 1991)
4.C.E. Williams et al., J Polym. Sci. Pt. C 17, 379 (1979)
5.A.Z. Akcasu et al., J. Polym. Sci. [B] 18, 863 (1980)
6.J.S. King et al., Macromolecules 18, 709 (1985)
7.J.S. Higgins, R.S. Stein, J. Appl. Cryst. 11, 346 (1978)
8.D.E. Groom, et al., (Particle Data Group), Eur. Phys. J. [C] 15, 1 (2000) and 2001 partial update for edition 2002 (URL, http,//pdg.lbl.gov)
9.G.D. Wignall, in Encyclopedia of Polymer Science and Engineering, vol. 10, 2nd edn. (Wiley, New York, 1987), pp. 112–184

158J.K. Krueger et al.
10.W. Schmatz, T. Springer, J. Schelten, K. Ibel, J. Appl. Cryst. 7, 96 (1974)
11.J.S. Higgins, H. Benoit, Polymers and Neutron Scattering (Clarendon Press, 1994)
12.R.M. Murphy, Curr. Opin. Biotechnol. 8, 25 (1997)
13.A.L. Papish, L.W. Tari, H.J. Vogel, Biophys. J. 83, 1455 (2002)
14.D.B. Heidorn et al., Biochemistry 28, 6757 (1989)
15.M. Ikura, G. Barbato, C.B. Klee, A. Bax, Cell Calcium 13, 391 (1992)
16.W.E. Meador, A.R. Means, F.A. Quiocho, Science 257, 1251 (1992)
17.J. Trewhella, J.K. Krueger, in Methods of Molecular Biology; vol. 173, H.J. Vogel (eds.) (Humana Press, 2001), pp. 137–160
18.J. Trewhella et al., Sci. Prog. 81, (1998)
19.J, Trewhella, Curr. Opin. Struct. Biol. 7, 702 (1997)
20.A. Maconnachie, Polymer 25, 1068 (1984)
21.L.D. Coyne, W.L. Wu, Polymer Commun. 30, 312 (1989)
22.G.D. Wignall, F.S. Bates, J. Appl. Cryst. 20, 28 (1987)
23.G.D. Wignall, in Physical Properties of Polymers, 3rd ed., ed. by J.E. Mark (Cambridge University Press, 2004), pp. 424–511
24.W.S. Dubner, J.M. Schultz, G.D. Wignall, J. Appl. Cryst. 23, 469 (1990)
25.D. Svergun et al., Proc. Natl. Acad. Sci. (USA) 95, 2267 (1998)
26.G.D. Wignall, in Polymer Properties Handbook, ed. by J.E. Mark (Cambridge University Press, 1996), pp. 299–310
27.K. Ibel, H.B. Stuhrmann, J. Mol. Biol. 93, 255 (1975)
28.P.B. Moore, J. Appl. Cryst. 14, 237 (1981)
29.G. Zaccai, B. Jacrot, Ann. Rev. Biophys. Bioeng. 12, 139 (1983)
30.D.L. Worcester, J. Appl. Cryst. 21, 669 (1988)
31.H.B. Stuhrmann, Zeit. fur Krist. 178, 208 (1987)
32.J. Schelten, Kerntechnik 14, 86 (1972)
33.J. Schelten, in Scattering Techniques Applied to Supramolecular and Nonequilibrium Systems, vol. 73, ed. by S.H. Chen, B. Chu, R. Nossal (Plenum Press, 1981), pp. 75–85
34.K. Ibel, J. Appl. Cryst. 9, 296 (1976)
35.Neutronenstreuexperimente am FRJ2 in J¨ulich (English and German texts are available from the Forschungszentrum, J¨ulich, 1997)
36.P. Lindner, R.P. May, P.A. Timmins, Physica B 180, 967 (1992)
37.C.J. Glinka et al., J. Appl. Cryst. 31, 430 (1998)
38.W.C. Koehler, Physica B and C 137, 320 (1986)
39.R.K. Abele, G.W. Allin, W.T. Clay, C.E. Fowler, M.K. Kopp, IEEE Transact. Nuc. Sci. 28, 811 (1981)
40.R.E. Ghosh, A.R. Rennie, J. Appl. Cryst. 32, 1157 (1999)
41.G.W. Lynn et al., J. Appl. Cryst. 36, 829 (2003)
42.J.B. Hayter, H. Mook, J. Appl. Cryst. 22, 35 (1989)
43.http://www.oecd.org/dsti/sti/s t/ms/prod/scattering.htm: A Twenty Years Look Forward at Neutron Scattering Facilities (1998)
44.G.D. Wignall et al., in Scattering Methods for the Investigation of Polymers, ed. by J. Kahovec (Wiley-VCH, Weinheim, 2002), pp. 185–200
45.http://www.sns.gov/
46.http://j-parc.jp/
47.http://www.isis.rl.ac.uk/targetstation2/
48.http://www.ess-europe.de/ess js/index.html
8 SANS from Biological Molecules |
159 |
49.P. Thiyagarajan et al., J. Appl. Cryst. 30, 280 (1997)
50.http://www.ill.fr
51.A. Guinier, G. Fournet, Small-Angle Scattering of X-rays (John Wiley, New York, 1955)
52.L.J. Magid, Colloids and Surfaces 19, 129 (1986)
53.J.B. Hayter, J. Penfold, Coll. Pol. Sci. 261, 1022 (1983)
54.V.F. Turchin, in Slow Neutrons (Sivan Press, Jerusalem, 1965), p. 16
55.B. Jacrot, G. Zaccai, Biopolymers 20, 2413 (1981)
56.P. Lindner, J. Appl. Cryst. 33, 807 (2000)
57.W.R. Krigbaum, F.R. Kugler, Biochemistry 9, 1216 (1970)
58.P. Lindner, F. Leclercq, P. Damay, Physica B 291, 152 (2000)
59.R.P. May, K. Ibel, J. Haas, J. Appl. Cryst. 15, 15 (1982)
60.J.R.D. Copley, J. Appl. Cryst. 21, 639 (1988)
61.W. Boyer, J.S. King, J. Appl. Cryst. 21, 818 (1988)
62.G.D. Wignall, D.K. Christen, V. Ramakrishnan, J. Appl. Cryst. 21, 438 (1988)
63.P.B. Moore, J. Appl. Cryst. 13, 168 (1980)
64.O. Glatter, J. Appl. Cryst. 10, 415 (1977)
65.V. Ramakrishnan, J. Appl. Cryst. 18, 42 (1985)
66.O. Glatter, O. Kratky, Small-Angle X-ray Scattering (Academic Press, New York, 1982)
67.G.D. Wignall, J. Appl. Cryst. 24, 479 (1991)
68.P.W. Schmidt, J. Appl. Cryst. 3, 257 (1970)
69.C.R. Wobbe, S. Mitra, V. Ramakrishnan, Biochemistry 23, 6565 (1984)
70.G.D. Wignall et al., J. Mol. Cryst. Liqu. Cryst. 180A, 25 (1990)
71.L. Fisher et al., J. Coll. Interface Sci. 123, 24 (1988)
72.L. Rayleigh, Proc. R. Soc. London Ser. A 84, 24 (1911)
73.O. Glatter, personal communication
74.J.S. Pederson, D. Posselt, K. Mortensen, J. Appl. Cryst. 23, 321 (1990)
75.P.S. Goyal, J.S. King, G.C. Summerfield, Polymer 24, 131 (1983)
76.J. Schelten, W. Schmatz, J. Appl. Cryst. 13, 385 (1980)
77.J.M. O’Reilly, D.M. Teegarden, G.D. Wignall, Macromolecules 18, 2747 (1985)
78.P.B. Moore, Methods Exper Phys 2, 337 (1982)
79.L.A. Feigin, D.I. Svergun, Structure Analysis by Small-Angle X-ray Scattering
(Plenum Press, New York and London, 1987)
80.D.I. Svergun, J. Appl. Cryst. 26, 258 (1993)
81.G.A. Olah, J. Trewhella, Biophys. J. 66, A311 (1994)
82.J.K. Zhao et al., J. Biol. Chem. 273, 30448 (1998)
83.http://www.sans.chem.umbc.edu/
84.http://www.embl-hamburg.de/ExternalInfo/Research/Sax/
85.F. Spinozzi, F. Carsughi, P. Mariani, J. Chem. Phys. 109, 10148 (1998)
86.H.B. Stuhrmann, Acta Cryst. A26, 297 (1970)
87.J.G. Grossmann et al., Biochemistry 32, 7360 (1993)
88.D.I. Svergun, M.H.J. Koch, I.N. Serdyuk, J. Mol. Biol. 240, 66 (1994)
89.D.I. Svergun et al., Proc. Natl Acad. Sci. USA 91, 11826 (1994)
90.D.I. Svergun et al., Acta Cryst. A 52, 419 (1996)
91.D.I. Svergun et al., J. Appl. Cryst. 30, 798 (1997)
92.D.I. Svergun, H. B. Stuhrmann, Acta Cryst. A 47, 736 (1991)
93.P. Chacon et al., Biophys. J. 74, 2760 (1998)
94.P. Chacon et al., J. Mol. Biol. 299, 1289 (2000)
160J.K. Krueger et al.
95.D.I. Svergun, Biophys. J. 76, 2879 (1999)
96.D.I. Svergun, M.V. Petoukhov, M.H.J. Koch, Biophys. J. 80, 2946 (2001)
97.D. Walther, F.E. Cohen, S. Doniach, J. Appl. Cryst. 33, 350 (2000)
98.W.T. Heller, J.K. Krueger, J. Trewhella, Biochemistry 42, 10579 (2003)
99.P. Debye, Ann. Phys. 46, 809 (1915)
100.D.B. Heidorn, J. Trewhella, Biochemistry 27, 909 (1988)
101.B.E. Kemp et al., Trends Biochem. Sci. 19, 440 (1994)
102.J.K. Krueger, R.C. Padre, J.T. Stull, J. Biol. Chem. 270, 16848 (1995)
103.P.J. Gallagher et al., J. Biol. Chem. 268, 26578 (1993)
104.B.E. Kemp, R.B. Pearson, Biochim. Biophys. Acta 1094, 67 (1991)
105.Y.S. Babu, C.E. Bugg, W.J. Cook, J. Mol. Biol. 204, 191 (1988)
106.G.M. Clore et al., Curr. Opin. Struct. Biol. 3, 838 (1993)
107.M. Ikura, Trends Biochem. Sci. 21, 14 (1996)
108.M. Ikura et al., Science 256, 632 (1992)
109.J.Goldberg, A.C. Nairn, J. Kuriyan, Cell 84, 875 (1996)
110.D.P. Fitzsimons et al., J. Biol. Chem. 267, 23903 (1992)
111.P.J. Kennelly et al., J. Biol. Chem. 262, 11958 (1987)
112.B.E. Kemp et al., J. Biol. Chem. 262, 2542 (1987)
113.J.K. Krueger et al., Biochemistry 36, 6017 (1997)
114.G.A. Olah, J. Trewhella, Biochemistry 33, 12800 (1994)
115.J.K. Krueger et al., Biochemistry 37, 13997 (1998)
116.M. Ikura, L.E. Kay, G. Barbato, S. Spera, A. Bax, FASEB J. 6, A403 (1992)
117.D.R. Knighton et al., Science 258, 130 (1992)
118.O.B. Peersen, T.S. Madsen, J.J. Falke, Protein Sci. 6, 794 (1997)
119.P.M. Bayley, W.A. Findlay, S.R. Martin, Protein Sci. 5, 1215 (1996)
120.J.K. Krueger et al., Biochemistry 37, 17810 (1998)
121.G.A. Olah et al., Biochemistry 32, 3649 (1993)
122.A. Persechini, K.J. Gansz, R.J. Paresi, Biochemistry 35, 224 (1996)
123.C.S. Tung, D.A. Walsh, J. Trewhella, J. Biol. Chem. 277, 12423 (2002)
124.R.S. Stein, in Neutron Scattering in the Nineties (IAEA, Vienna, 1985), p. 335
125.http://www.ill.fr/Yellowbook/deuteration

9
Small Angle Neutron Scattering
from Proteins, Nucleic Acids, and Viruses
S. Krueger, U.A. Perez-Salas, S.K. Gregurick, D. Kuzmanovic
9.1 Introduction
This chapter will focus on SANS applications to complex biological macromolecules such as proteins, nucleic acids, viruses, micelles, and vesicles. Because of its sensitivity to the biologically important light elements such as H, C, N, and O, SANS can provide unique information on the structure and function of biological macromolecules. Recent advances in biochemistry, crystallography and structural NMR have made it possible to prepare greater quantities of deuterium-labeled proteins and to determine an ever-increasing number of high-resolution structures. Thus, SANS has also come into wider use as a complementary tool for comparing the structures in crystal and solution phases and for elucidating the unresolved regions in a crystal structure. Since the measurements are performed in solution, SANS gives unique structural information under conditions that more closely mimic the molecule’s natural environment, and thus can provide critical insights in a number of bioengineering areas.
The SANS experimental method has been described previously. Detailed information on SANS from biological macromolecules can be found in this book and in several review articles [1–3]. In the case of complex systems such as viruses, nucleic acids, and proteins, it is often far easier to obtain data than to interpret what the data mean. One simple, model-independent analysis of the scattered intensity, I(Q), that is normally performed is the Guinier approximation [4], given by,
I(Q) = I(0) exp − |
QR |
2 |
, |
|
g |
|
(9.1) |
||
3 |
|
where Rg is the radius of gyration, I(0) is the forward scattered intensity and Q = 4π sin(θ)/λ, where λ is the neutron wavelength and 2θ is the scattering angle. This approximation is only valid in the region where QRg ≈ 1. A realspace representation of the data can be obtained from the distance distribution function, P (r), which is related to I(Q) by
