
Neutron Scattering in Biology - Fitter Gutberlet and Katsaras
.pdf
40 C.C. Wilson et al.
Fig. 2.5. Detergent structure present in crystals of the peripheral light-harvesting complex of the purple bacteria Rhodopseudomoas acidophila strain 10050 determined by neutron crystallography at 12 ˚A resolution
general “small” molecules, where the main interest is in understanding their conformation and interactions, aiming to project these properties into understanding their interactions with macromolecules and hence their function.
One of the largest such molecules subjected to high resolution single crystal neutron di raction is cyclosporin A, an immunosuppresant drug with wide clinical application [45]. Stable refinements of this structure were obtained from data collected on H3A at Brookhaven on a 20 mm3 sample, in spite of a low data to parameter ratio of just 2.3. In addition to defining fully the hydrogen atom geometry in this large organic molecule (C62H111N311O12·H2O; 199 atoms in the asymmetric unit), the neutron study revealed the presence of a bound, ordered water molecule – an ordered solvent interaction.
In general, neutron single crystal di raction is of enormous value in the study of pharmaceuticals, where many drug molecules crystallise with unit cells in the accessible cell range up to 104 ˚A3. Detailed neutron data can be vital to the understanding of molecular conformation, especially with regard to the often very small energy di erences between active and inactive polymorphs. Neutrons also sample the bulk of such materials, again vital in the study of polymorphism in relation to production processes.
2 Neutron Di raction and Protein Crystallography |
41 |
2.5 Recent Developments and Future Prospects
The recent developments in studying biological structures at high resolution with neutrons have focused around the use of neutron image plates along with modified Laue methods [46]. These developments, including the Japanese BIX instrument and LADI at the ILL, are discussed above and will be discussed in more detail by other contributors to this volume, as well as the potential for high impact of these methods in the field of biomolecular crystallography.
References
1.C.C. Wilson, Single Crystal Neutron Di raction from Molecular Materials
(World Scientific, Singapore, 2000)
2.http://www.ill.fr/
3.C. Wilkinson, M.S. Lehmann, Nucl. Inst. Methods A310, 411–415 (1991)
4.http://www.isis.rl.ac.uk/
5.X.M. Li, P. Langan, R. Bau, I. Tsyba, F.E. Jenney, M.W.W. Adams, B.P. Schoenborn, Acta Cryst. D60, 200–202 (2004)
6.B.L. Hanson, P. Langan, A.K. Katz, X.M. Li, J.M. Harp, J.P. Glusker, B.P. Schoenborn, G.J. Bunick, Acta Cryst. D60, 241–249 (2004)
7.N. Niimura, Y. Minezaki, T. Nonaka, J.C. Castagna, F. Cipriani, P. Hoghoj, M.S. Lehmann, C. Wilkinson, Nature Struct. Biol. 4, 909–914 (1997); J.R. Helliwell, Nature Struct. Biol. 4, 874–876 (1997)
8.S. Fujiwara, Y. Karasawa, I. Tanaka, Y. Minezaki, Y. Yonezawa, N. Niimura, Physica B 241, 207–209 (1997)
9.Recent developments are discussed in S. Tazaki, K. Neriishi, K. Takahashi, M. Etoh, Y. Karasawa, S. Kumazawa, N. Niimura, Nucl. Inst. Methods A424, 20–25 (1999)
10.http://www.isis.rl.ac.uk/TargetStation2/
11.http://www.sns.gov/
12.http://www.j-parc.jp/
13.J. Kjems, A.D. Taylor, J.L. Finney, H. Lengeler, U. Steigenberger, ESS: A Next Generation Neutron Source for Europe, Volume I: The European Spallation Source; Volume II: The scientific case (ESS Council, Roskilde, 1997)
14.W. Jauch, M.S. Lehmann, L. Sj¨olin, C. Wilkinson, C.C. Wilson, ILL Internal Report, ILL97/JA19T (Institut Laue-Langevin, Grenoble, 1997)
15.C.C. Wilson, in Implications of Molecular and Materials Structure for New Technologies, ed. by J.A.K. Howard, F.H. Allen, G.P. Shields. NATO Science Series E: vol 360 (Kluwer, Dordrecht, 1999), pp. 11–21
16.C.C. Wilson, (1998) http://www.rsc.org/pdf/forwardlook/neutrongrp.pdf.
17.R.B. Knott, B.P. Schoenborn, in Neutrons in Biology, ed. by B.P. Schoenborn, R.B. Knott (Plenum, New York, 1996), pp. 1–15
18.I. Tsyba, R. Bau, Chemtracts 15, 233–257 (2002)
19.F. Shu, V. Ramakrishnan, B.P. Schoenborn, Proc. Natl. Acad. Sci. USA 97, 3872–3877 (2000)
20.A. Wlodawer, L. Sj¨olin, Biochemistry 22, 2720–2728 (1983)
42C.C. Wilson et al.
21.G.A. Bentley, E.D. Duee, S.A. Mason, A.C.J. Nunes, Chim. Phys. 76, 817–821 (1979); G.A. Bentley, M. Delepierre, C.M. Dobson, R.E. Wedin, S.A. Mason, F.M.J. Poulsen, J. Mol. Biol. 170, 243–247 (1983); S.A. Mason, G.A. Bentley, G.J. McIntyre, in Neutrons in Biology, ed. by B.P. Schoenborn (Plenum, New York, 1984), pp. 323–334
22.M.S. Lehmann, R.F.D. Stansfield, Biochemistry 28, 7028–7033 (1989)
23.M.M. Teeter, A.A. Kossiako , in Neutrons in Biology, ed. by B.P. Schoenborn (Plenum, New York, 1984), pp. 335–348
24.H.F.J. Savage, P.F. Lindley, J.L. Finney, P.A. Timmins, Acta Cryst. B43, 280–295 (1987)
25.J.P. Bouquiere, J.L. Finney, M.S. Lehmann, P.F. Lindley, H.F.J. Savage, Acta Cryst. B49, 79–89 (1993)
26.F.M. Moore, B.T.M. Willis, D. Crowfoot-Hodgkin, Nature 214, 130–133 (1967)
27.B.P. Schoenborn, Nature 224, 143–146 (1969)
28.J.C. Hanson, B.P. Schoenborn, J. Mol. Biol. 153, 117–146 (1981)
29.S.E.V. Phillips, B.P. Schoenborn, Nature 292, 81–82 (1981)
30.A.A. Kossiako , S.A. Spencer, Biochemistry 20, 6462–6474 (1981)
31.A.A. Kossiako , S.A. Spencer, Nature 288, 414–416 (1980)
32.L. Coates, P.T. Erskine, S.P. Wood, D.A. Myles, J.B. Cooper, Biochemistry 40, 13149–13157 (2001)
33.J. Vojtechovsky, K. Chu, J. Berendzen, R.M. Sweet, I. Schlichting, Biophys.
J.77, 2153–2174 (1999); A.E. Miele, L. Federici, G. Sciara, F. Draghi,
M.Brunori, B. Vallone, Acta Cryst. D59, 982–988 (2003)
34.A.A. Kossiako , S. Shteyn, Nature 311, 582–583 (1984)
35.H.F.J. Savage, Biophys. J. 50, 947–965 (1986); H.F.J. Savage, Biophys. J. 50, 967–980 (1986)
36.S.E.V. Phillips, B.P. Schoenborn, Nature 292, 81–82 (1981); N.V. Raghavan, B.P. Schoenborn, in Neutrons in Biology, ed. by B.P. Schoenborn, (Plenum, New York, 1984), pp. 247–259; M.M. Teeter, Proc. Natl. Acad. Sci. USA 81, 6014–6018 (1984); H.F.J. Savage, A. Wlodawer, Methods Enzymol. 127, 162–183 (1986)
37.B.P. Schoenborn, J. Mol. Biol. 201, 741–749 (1988); X. Cheng, B.P. Schoenborn, Acta Cryst. B46, 195–208 (1990); A.A. Kossiako , M.D. Sintchak,
J.Shpungin, L.G. Presta, Proteins: Struct., Funct. and Gen., 12, 223 (1992)
38.M.P. Blakeley, M. Cianci, J.R. Helliwell, P.J. Rizkallah, Chem. Soc. Rev., 33, 548–557 (2004)
39.A.A. Kossiako , Nature 296, 713–721 (1983)
40.T. Chatake, K. Kurihara, I. Tanaka, I. Tsyba, R. Bau, F.E. Jenney Jr., M.W. Adams, N. Niimura, Acta Cryst. D60, 1364–1373 (2004)
41.M. Roth, A. Lewit-Bentley, H. Michel, J. Deisenhofer, R. Huber, D. Oesterhelt, Nature 340, 659–662 (1989)
42.P.A. Timmins, B. Poliks, L.J. Banaszak, Science, 257, 652–655 (1992)
43.G. Zaccai, J.K. Blasie, B.P. Schoenborn, Proc. Natl. Acad. Sci. USA, 72, 376–380 (1975); J.F. Pardon, D.L. Worcester, J.C. Wooley, K. Tatchell, K.E. van Holde, B.M. Richards, Nucleic Acids Res. 2, 2163–2176 (1975); D.L. Worcester, N.P. Franks, J. Mol. Biol. 199, 359–378 (1976)
44.S.M. Prince, T.D. Howard, D.A.A. Myles, C. Wilkinson, M.Z. Papiz, A.A. Freer, R.J. Cogdell, N.W. Isaacs, J. Mol. Biol. 326, 307–315 (2003)
45.R.B. Knott, J. Schefer, B.P. Schoenborn, Acta Cryst. C46, 1528–1533 (1990)
46.J.R. Helliwell, Nature Struct. Biol. 4, 874–876 (1997)

3
Neutron Protein Crystallography:
Hydrogen and Hydration in Proteins
N. Niimura
3.1 Introduction
The three-dimensional structure determination of biological macromolecules such as proteins and nucleic acids by X-ray crystallography has improved our understanding of many of the mysteries involved in life processes. At the same time, these results have clearly suggested that hydrogen and water molecules around proteins and nucleic acids play a very important role in many physiological functions. However, since it is very hard to determine positions of hydrogen atoms in protein molecules using X-rays, a detailed discussion of protonation and hydration sites is often very speculative upon so far. In contrast, neutron di raction provides an experimental method of locating hydrogen atoms much more precise. Despite this quality, the examples of protein structure determination by neutrons are relatively low since the requested sample size of the protein crystals is larger and it takes a considerable amount of time to collect a su cient number of Bragg reflections.
The recent development of a neutron imaging plate (NIP) became a breakthrough in the application of neutron protein crystallography (NPC) [1–3]. Its first application of NIP was the structure determination of tetragonal hen-egg-white lysozyme using the quasi-Laue di ractometer, LADI at the Institute Laue-Langevin (ILL) in Grenoble [4]. In the Japan Atomic Energy Research Institute (JAERI), several high-resolution neutron di ractometers (BIX-type di ractometers) dedicated to biological macromolecules have been constructed, which exploit NIP using monochromatized neutron beam [5–9]. Detailed descriptions on NIP and the BIX-type di ractometers are given in the contribution by Wilson et al. in this volume and in the original papers, respectively.
The general subject of NPC has been already reviewed by several authors [10–17]. Their articles are recommended and helpful in understanding the historical background of this approach. In this chapter, several topics of NPC relevant to hydrogen positions and hydration in proteins, obtained using BIX-type di ractometers will be presented. The proteins that will be treated

44 N. Niimura
in the present chapter are myoglobin (Mb) [18], wild type rubredoxin (Rb- w) [19], a mutant form of rubredoxin (Rb-m) [20], hen-egg-white lysozyme (HEWL) at pH 4.9 [21] and cubic porcine insulin [22].
3.2 Complementarity of Neutrons and X-rays
The distinctive features of neutrons are summarized as follows: (i) Since the neutron scattering lengths densities of hydrogen and deuterium are comparable to those of other elements, they are easily observed by neutrons. The X-ray atomic scattering factor of hydrogen is much less and hydrogen is hard to be observed by X-rays. (ii) Since a proton (H+) has no electrons, it can not be seen by X-rays, on the contrary it can be observed by neutrons. Using heavy water (D2O) in neutron di ractometry for the crystallization of proteins H+ can be replaced by D+ and observed by neutrons. Mobile hydrogen atoms can be distinguished if they are replaced by deuterium as the neutron scattering lengths densities of hydrogen and deuterium are di erent. Table 3.1 shows the neutron scattering lengths densities and X-ray atomic scattering factors of some of the elements which constitute proteins.
3.2.1 Refinement of Hydrogen Positions
Since the neutron scattering lengths densities of hydrogen and deuterium are comparable to those of other elements, in neutron protein crystallography they are not only identified but also their positions can be refined like other elements, such as carbon, nitrogen and so on. Several examples are shown in Fig. 3.1. Figure 3.1a shows a 2|Fo| − |Fc| nuclear density map of Phe48 of wild type rubredoxin at 1.5 ˚A resolution [19]. The neutron scattering lengths density of the hydrogen atoms is negative, while deuterium, carbon, nitrogen, and oxygen atoms all have positive neutron scattering lengths densities. In the map shown, the hydrogen atoms bound to carbon atoms are clearly visible. Their negative neutron scattering length density is clearly separated from the
Table 3.1. Neutron scattering lengths and X-ray atomic scattering factors
|
neutron |
X-ray |
atom |
bcoh (10−12 cm) |
fX-ray (10−12 cm) |
D+ |
0.67 |
0 |
H |
−0.37 |
0.28 |
D |
0.67 |
0.28 |
C |
0.67 |
1.69 |
N |
0.94 |
1.97 |
O |
0.58 |
2.25 |
S |
0.29 |
4.48 |
3 Neutron Protein Crystallography |
45 |
(a) |
(b) |
(c) |
Fig. 3.1. 2|Fo|−|Fc| nuclear density maps around. (a) Phe48, (b) Tyr12, (c) Trp36
positive densities of the carbon atoms. Figure 3.1b shows a similar nuclear density map for Tyr12 of wild type rubredoxin at 1.5 ˚A resolution [19]. The hydrogen atoms produce a large amount of incoherent scattering, which results in an undesirably high level of background radiation in the neutron di raction experiment. This e ect can be partially overcome either by growing crystals from, or by soaking the crystals in, D2O solutions. This treatment leads to the replacement of hydrogen atoms bound to nitrogen and oxygen (exchangeable hydrogens) by deuterium, as well as of the hydrogen atoms of the solvent molecules in the crystal, without modification of the overall structure of the macromolecule. In Fig. 3.1b, the density contours of the hydrogen atom of the O–H bond in Tyr12 have a positive value, thus it can be concluded that it has been replaced by a deuterium atom. Figure 3.1c shows the 2|Fo| − |Fc| nuclear density of Trp36 of wild type rubredoxin at 1.5 ˚A resolution [19]. It is seen that the N–H bond of the indole ring has a positive density value, i.e., it has become an N–D bond.
3.2.2 Hydrogen Atoms Which Cannot be Predicted Stereochemically
The positions of hydrogen atoms covalently bound to carbon atoms can be calculated stereochemically based on the coordinates of carbon and nitrogen atoms determined by high resolution X-ray crystal structure analysis. However, the positions of some hydrogen atoms covalently bonded to carbon atoms are di cult to be calculated stereochemically, and if hydrogen atoms bound to oxygen, nitrogen, and sulfur atoms become protons, they are impossible to be identified and refined because protons have no electrons scattering X-rays. These hydrogen atoms and protons are summarized in Table 3.2. Neutrons can identify and refine these hydrogen positions.

46 N. Niimura
Table 3.2. Hydrogen atoms (protons) in proteins
|
|
|
detection of hydrogen |
|
|
|
|
|
positions |
|
|
|
|
|
|
chemical |
|
X-ray |
stereo- |
functional group |
structure |
residues |
analysis |
chemically |
|
|
|
|
|
aromatic ring |
Φ-H, Φ-D |
Phe, Tyr, Trp, His |
possible |
possible |
alkyl group |
–CH–, –CH2– |
all residues |
possible |
possible |
(except methyl) |
|
|
|
|
peptide group |
–ND– |
all residues |
possible |
possible |
and –ND group |
|
except His |
|
|
methyl group |
–CH3 |
Ala, Ile, Leu, Met, |
hard |
hard |
|
|
Thr, Val |
|
|
protonated amino |
–ND3 |
N-terminus, Lys |
hard |
hard |
group |
|
|
|
|
hydroxyl group |
–OD |
Ser, Thr, Tyr |
hard |
impossible |
protonated |
–COOD |
C-terminus, Asp, |
impossible/hard impossible |
|
carboxyl group |
|
Glu |
|
|
amino group |
–ND2 |
Arg, Asn, Gln |
hard |
impossible |
sulfhydryl group |
–SD |
Cys |
hard |
impossible |
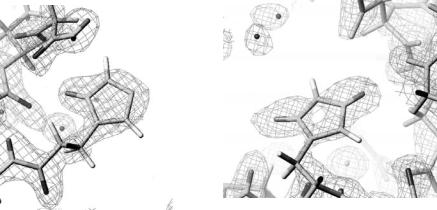
The hydrogen atoms in methyl groups can sometimes be significantly o their predicted positions because of free rotation around the C–C bonds. Consequently, if their precise positions are required they should be determined by neutron di raction experiments. Figure 3.2 shows examples of some methyl hydrogen atoms in wild type rubredoxin determined from neutron di raction data [19].
In X-ray protein crystallography sometimes it is very di cult to distinguish the nitrogen and oxygen atoms in Asn and Gln. In neutron protein crystallography such a di culty does not occur on replacement of hydrogen of the amino groups by deuterium atoms (–ND2) which are identified very easily. Figure 3.3 shows the 2|Fo| − |Fc| Fourier map of Asn21 in the mutant form of rubredoxin [20].
The protonation and deprotonation states of the two nitrogen atoms (Nπ, Nτ) in the imidazole ring of histidine are often very important pieces of information that need to be known in order to fully understand the function of certain enzymes, as well as the metal complexation behavior of certain proteins. This information can be obtained from neutron di raction. Figures 3.4a, b show the 2|Fo| − |Fc| nuclear density maps of the His5 and His10 residues, respectively, of the B-chain of cubic porcine insulin at 1.6 ˚A resolution [22]. The protein is a hetero-dimer, composed of an A-chain and a B-chain. For His5 of the B-chain, Nπ is protonated and Nτ is deprotonated. In contrast, for His10, both Nπ and Nτ are protonated. This means that His5 is electronically neutral while His10 is positively charged.

|
3 |
Neutron Protein Crystallography |
47 |
|
(a) AIa43 |
(b) |
IIe7 |
|
|
|
Cb |
Cd |
Cg2 |
|
|
Cg1 |
|
||
Cb
Ca
Ca
Fig. 3.2. |Fo| − |Fc| omit map of the hydrogen atoms around the residues Ala43 (a), Ile7 (b)
−ND2
=O
Hβ2 Hβ1
Asn21
Fig. 3.3. The 2|Fo| − |Fc| Fourier map of Asn21 in the mutant form of rubredoxin
As mentioned earlier, polar hydrogen atoms, like those of N–H and O–H bonds, can be exchanged by deuterium if the protein crystal is soaked in a D2O bu er. In contrast, hydrogen atoms bound to carbon are normally not exchangeable. An exception is the hydrogen atom bonded to the Cε1 carbon atom of histidine. The Cε1–H of the imidazole group is the most acidic C–H bond found in amino acids [23]. Therefore this hydrogen atom is in principle exchangeable, depending on its environment. Direct experimental evidence for this behavior was found in metmyoglobin. Figure 3.5 shows the nuclear density map for His97 in this protein [18].
48 N. Niimura
(a) |
(b) |
Nπ Nτ
B Chain His 5 |
B Chain His 10 |
Fig. 3.4. 2|Fo| − |Fc| nuclear density map of (a) His5 and (b) His10 in the B-chain of cubic porcine insulin
The Hε1-atom clearly shows positive neutron density contours near Cε1, indicating an exchange of the H atoms of the C–H bond to deuterium. An occupancy refinement yields the ratio 80% D/20% H. The nitrogen atom Nε2- atom is also deuterated (occupancy: 65% D). The alternative conformation, obtained by rotating the imidazole ring by 180◦ around the Cβ–Cγ axis, cannot explain this finding since the Hδ2-atom shows a full hydrogen occupancy (negative density in Fig. 3.5). His97 is located on the so-called proximal side of the heme plane (the ligand binding position is on the other site of the heme plane, the so-called distal side). To our knowledge, this is the first time neutron di raction has been used to verify the acidic character of the H 1 atom of His97 in myoglobin. In hen-egg-white lysozyme, a similar conclusion was reported recently [24].
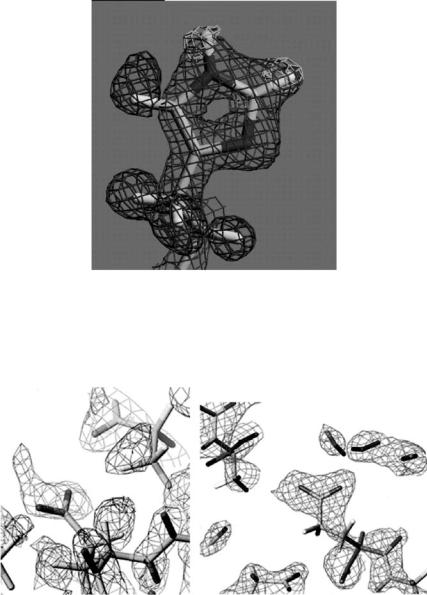
In the proposed mechanism of the reaction of lysozyme with oligosaccharides, consideration was given to the fact that the enzyme activity is maximal at pH 5 and is less active at pH 7. It is postulated that at pH 5, the carboxylate group of Glu35 is protonated, and it is this proton that is transferred to the oxygen atom on the bound substrate (sugar) during the hydrolysis process. During the reaction, another acidic residue Asp52, remains in its dissociated state [34]. In order to elucidate the role of hydrogen atoms in this reaction, neutron di raction experiments of hen-egg-white lysozyme, crystals of which have been grown at di erent pH’s (specifically, 4.9 [21] and 7.0 [4]), have been carried out. The detailed procedures of these neutron structure analyzes are given in [4, 21]. The results shown in Fig. 3.6a, b show the 2|Fo|−|Fc| nuclear density map around the carboxylate group of Glu35 at pH 4.9 (Fig. 3.6a) and pH 7.0 (Fig. 3.6b). As indicated by an arrow in Fig. 3.6a,
3 Neutron Protein Crystallography |
49 |
65% D
80% D/20% H
Cεl |
Hεl |
Fig. 3.5. Nuclear density maps of His97 in myoglobin: 2|Fo| − |Fc| map (positive) (dark gray); |Fo| − |Fc| omit map (positive) (light gray); |Fo| − |Fc| omit map (negative) (gray). All H(D)-atoms were omitted for the calculation of the Fc and jc for the omit-map
(a) |
(b) |
Asp52
E350ε2
E350ε1
Glu35
pH4.9 |
pH7.0 |
Fig. 3.6. 2|Fo| − |Fc| nuclear density map around the carboxylate group of Glu35 at pH 4.9 (a), and Fig. 3.15(b) at pH 7.0
