
Astruc D. - Modern arene chemistry (2002)(en)
.pdf
10.2 The DoM Reaction as a Methodological Tool 333
Scheme 3. DoM chemistry generalizations.
ditions for metalation chemistry B follow chacun `a son gouˆt principles: the chemist interested in small-scale reactions refers to them as mild, while the process chemist is concerned about the engineering di culties and energy demands of anhydrous, low temperature reactions. The complementarity of DoM to aromatic Eþ substitution C is reinforced by the normally harsh, Lewis-acid mediated conditions, and the non-regioselective nature of various reaction types, which complicate separation and isolation procedures. Many DMGs are conducive to sequential ortho,ortho0-metalation DI, thereby allowing the establishment of 1,2,3- substitution patterns. Alternatively, this pattern may be established by taking advantage of the synergism of two DMGs to in-between metalation DII or by the anionic ortho-Fries rearrangement E. Iterative DoM sequences F are also possible, sometimes in one-pot, which o er unique solutions to the introduction of di cult aryl substitution patterns. Unusually substituted systems may also be derived by silicon protection tactics of both aromatic and tolyl CaH acidic sites G [9a].
10.2.1
The N-Cumyl Carboxamide, Sulfonamide, and O-Carbamate DMGs
The stalwart diethyl carboxamide, while a powerful DMG, su ers from treacherous hydrolytic stability, a consequence which has recently been overcome by the development of the N-cumyl carboxamide (Scheme 4) [11]. Thus, the readily available 1 undergoes smooth metalation and acceptance of a variety of electrophiles; if DMF is used, the intermediate hydroxyphthalimidine 2 may be further metalated and subjected to electrophile quench to af-

334 10 The Directed ortho Metalation Reaction -- A Point of Departure for New Synthetic Aromatic Chemistry
Scheme 4. Synthetic utility of the N-cumyl carboxamide DMG.
ford 4-substituted products 3, conversion of which into 4 establishes a new route to such apparently rare systems, which are normally obtained by classical, non-regioselective Eþ substitution routes, 5 ! 6.
Similarly, the N-cumyl benzene sulfonamide 7 (Scheme 5) undergoes metalation–diverse electrophile quench reactions; its quenching with DMF leads to 8, which, after OTMS protection, may be further metalated and quenched with iodine to give 10; the latter is transformed under mild conditions into new benzothiazole derivatives 11 and 12 [12].
The O-carbamate, the most powerful of DMGs in competition experiments [13], is similarly plagued by stability as the N,N-diethyl derivative 13 (Scheme 6). However, the N-cumyl- N-methyl counterpart 14 undergoes unproblematic metalation–electrophile quench to give 15 and anionic Fries rearrangement (16) reactions. The very mild conditions for hydrolysis to 17, and hence to 18, as well as to 19 bode well for further useful chemistry of the N-cumyl DMG in context of more substituted aromatics [14].
10.2.2
The Lithio Carboxylate and Carboxylate Ester DMGs
Recent results from the laboratories of Mortier o er the potential of the carboxylate DMG as the simplest solution for the preparation of benzoic acid derivatives (Scheme 7) [15]. In order to minimize the expected ketonic products, the metalation 20 ! 21 must be carried out at 90 C, but thus leads to useful yields of products 22. Based on experiments on metainterrelated DMG systems and competition with other DMGs, 23–26 also o er application possibilities. Of similar synthetic potential is the work of Kondo (Scheme 8) [16], which

10.2 The DoM Reaction as a Methodological Tool 335
Scheme 5. The N-cumyl sulfonamide DMG.
Scheme 6. The N-cumyl carbamate DMG.
demonstrates DoM chemistry 27 ! 28 using TMP-zincate bases for not only amide but also ester and nitrile DMGs to give high yields of, as yet few, products 29. Negishi cross-coupling chemistry 30 ! 31 has also been achieved. Combined in situ LiTMP/borate and LiTMP/ silane base-electrophile species may be similarly used for DoM chemistry of ester DMG systems [17].

336 10 The Directed ortho Metalation Reaction -- A Point of Departure for New Synthetic Aromatic Chemistry
Scheme 7. The COO DMG.
Scheme 8. DoM chemistry with TMP-zincate as base.
10.2.3
The Di-tert-Butyl Phosphine Oxide DMG
The current activity in the area of enantioselective organometallic catalysis using phosphorus-based DMGs stimulated activity to improve the existing repertoire of P-DMGs. Phenyl di-tert-butyl phosphine oxide 33 (Scheme 9), chosen for its stability to alkyllithiums and readily prepared from 32, shows excellent metalation traits to give, after electrophile quench, a variety of interesting aromatic phosphorus derivatives 34, including di-phosphorus and boron-phosphorus compounds [18]. The di culty of DMG removal plagues this methodology, a factor that also surfaces in its use in heterocyclic DoM chemistry (Scheme 16), and thus should prompt further studies.

10.3 Heteroaromatic Directed ortho Metalation (HetDoM) in Methodological Practice 337
Scheme 9. The di-tert-butyl phosphine oxide DMG.
10.3
Heteroaromatic Directed ortho Metalation (HetDoM) in Methodological Practice
The routine requirement of substituted heteroaromatics, varying from the traditionally defined p-excessive (furans, thiophenes, pyrroles, and their benzo analogues) and p-deficient (pyridines and benzo analogues) and their multitudes of diand higher-heteroatom containing relatives, prompts continuing intense studies to explore the scope and limitations of HetDoM chemistries. Although the former derivatives are electrophilically active, their sensitivity to further decomposition pathways as a result of excessive reactivity, at times, limits such chemistry. In contrast, the pyridines, quinolines, and related p-deficient systems are, of course, recalcitrant to electrophilic substitution, a fact which causes either modification of substituted commercial products by classical reactions or de novo ring synthesis approaches to obtain polysubstituted systems. While numerous workers have contributed to DoM chemistry of p-excessives [19], the Que´guiner school [9e, 9f ] has been the major force in contributions to DoM in the pyridine and diazine areas, which will have a major impact in devising new routes to substituted derivatives that are not readily prepared by conventional means.
10.3.1
p-Excessive Heteroaromatic Directed ortho Metalation (HetDoM)
10.3.1.1 Furans and Thiophenes
In metalation of the p-excessive furans and thiophenes, consideration must be given to not only the DMG e ect but also the inherent acidity of the 2-,(5-)hydrogens (Scheme 10) [19, 20].
While the standard carbon-based DMGs have received substantial application in these systems, the heteroatom-based groups have enjoyed limited use with the exception of the extensive studies on sulfonamides (Scheme 13). Although amide and oxazoline DMGs have

338 10 The Directed ortho Metalation Reaction -- A Point of Departure for New Synthetic Aromatic Chemistry
Scheme 10. DoM reactivity of furans and thiophenes.
found considerable application, the carboxylic acid DMG in furans and thiophenes and their benzo analogues are arguably the most useful for further manipulation (Scheme 11) [21]. An instructive illustration is the use of furan carboxylic acid 35 in the synthesis of sesquiterpenoid 37 via the diester 36, which is prepared by condensation of an anhydride with dianion of 35, the basic character of which appears to have minimal detrimental e ects (Scheme 12) [22].
Scheme 11. The COO DMG in furans and thiophenes.
Scheme 12. Application of the COO DMG in furans.
Thiophene sulfonamide DoM chemistry is by far the most highly explored in view of the agrochemical significance of the sulfonylurea herbicides [23]. Potential for regioselective deprotonation as a function of kinetic or thermodynamic control 38 may lead to 3- or 5-

10.3 Heteroaromatic Directed ortho Metalation (HetDoM) in Methodological Practice 339
substituted products 39 ! 40 or 41, which can be further manipulated hydrolytically at the tert-butyl sulfonamide function (Scheme 13) [24]. C-5 functionalization via Weinreb amides to 42 is also feasible.
Scheme 13. DoM reactivity of the SO2N R DMG in thiophenes.
10.3.1.2 Indoles
While pyrrole DoM chemistry is still a relatively unexplored area [25], the corresponding indoles, especially with respect to N-DMGs (Scheme 14), constitute a rich area of metalation exploitation [26].
The introduction of a 2-DMG or the presence of a 3-DMG allows further metalation chemistry at the respective alternative positions on the pyrrole ring (Scheme 15). The chemistry of the 3-amides and 3-carboxylic acids 43 and 44 is illustrative of the potential for
Scheme 14. N-DMGs for DoM of indoles.

34010 The Directed ortho Metalation Reaction -- A Point of Departure for New Synthetic Aromatic Chemistry
formation of indole 2,3-substituted products (Scheme 15) [27]. Access to 7-substituted indoles via metalation chemistry has been achieved via indolines [28] and, more recently, specially designed N-DMGs such as N-COC(Et)3 [29], and N-P(O)(tBu)2 (Scheme 16) [30]. In the last example, although the starting material 46 is easily prepared from indole (45) and highly regioselective 2- (48) or 7- (47) deprotonation can be achieved by judicious choice of conditions, the di culty of removing the phosphite group remains.
Scheme 15. 3-Amides and 3-carboxylic acids as DMGs in DoM of indoles for the synthesis of 2,3-substituted indoles.
Scheme 16. The N-P(O)(t-Bu)2 DMG for the synthesis of 7-substituted indoles.
10.3 Heteroaromatic Directed ortho Metalation (HetDoM) in Methodological Practice 341
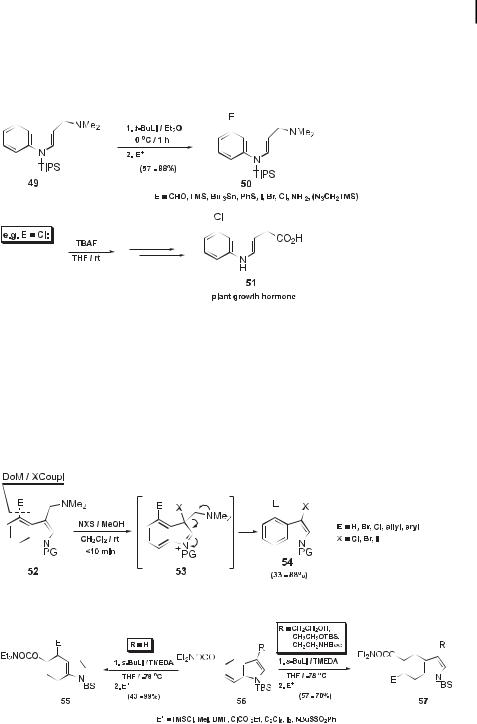
The discovery by Iwao that gramine bearing the large N-silicon functionality TIPS, 49, undergoes 4-deprotonation to give, after electrophile quench, products 50 (Scheme 17) [31] is of great synthetic consequence for the preparation of di cult to access 4-substituted indoles, e.g. 51.
Scheme 17. 4-Deprotonation of gramine bearing a bulky N-silyl functionality.
Furthermore, the vintage observation [32] of retro-Mannich reactivity of gramines has allowed the development of a new route to 3,4-disubstituted indoles, especially dihalogenated derivatives 52 ! 53 ! 54 (Scheme 18) [33]. In order to open the door to DoM reactivity studies in the benzo ring component of indoles, the 5-O-carbamate 56 has recently been systematically studied (Scheme 19) [34]. The absence or presence of 3-substitution, no matter what size, allows, respectively, 4- (55) and 6- (57) derivatization of indoles, including tryptophols and tryptamines [35].
Scheme 18. 3,4-Substituted indoles via DoM-retro Mannich sequence.
Scheme 19. DoM benzenoid ring functionalization of indole 5-O-carbamate.

34210 The Directed ortho Metalation Reaction -- A Point of Departure for New Synthetic Aromatic Chemistry
10.3.2
p-Deficient Heteroaromatic Directed ortho Metalation (HetDoM)
10.3.2.1 Pyridines
DoM of pyridines may be compromised by coordinatively driven addition of RLi and LiNR2 reagents. The synthetic value of such reactivity for substituted pyridines 57 ! 58 is undeniable (Scheme 20) [36] and may be considered at times complementary to HetDoM (Scheme 21) [9d, 9e, 20d, 37]. As evidenced from methodological use as well as in total synthesis endeavors [9d, 9e], amide and halogen DMGs are most valuable for substituted pyridine synthesis. The recent addition of the CO2 DMG may have future impact [38].
Scheme 20. Nucleophilic addition to pyridines.
Scheme 21. DMGs in DoM chemistry of pyridines.
Among the methodological studies [9d, 9e, 20d], the rich chemistry of carbinolamine alkoxide DMGs 59–63 (Scheme 22) [39], while not explored with regard to the scope of electrophile introduction, predicts access to highly functionalized systems. Similarly as yet unexplored but of foreseeable value for 3,5-disubstituted patterns is the use of the O-carbamate DMG, 64 ! 65 (Scheme 23) [40]. Thus, in a prototype sequence, metalation–electrophile
