
Astruc D. - Modern arene chemistry (2002)(en)
.pdf
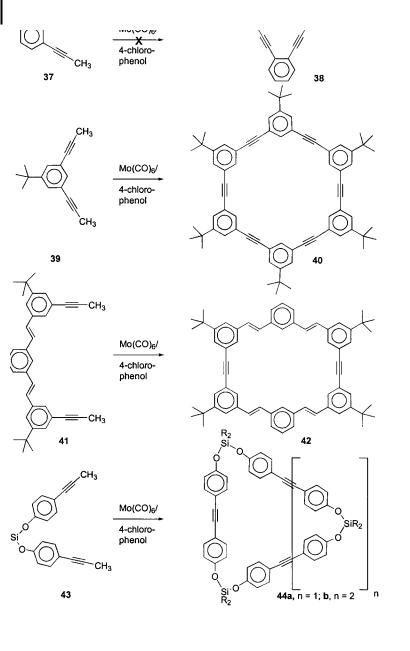
7.2 Syntheses 223
The yields are lower in these more di cult cases. It was demonstrated that the metathesis conditions do not exert any oxidative stress on the substrates. Dimerization of 27 furnished spectroscopically pure 32 in 99 % yield after precipitation from methanol. Under oxidative stress, the ferrocene nucleus would have been oxidized and paramagnetic material would have been isolated.
While butadiene units and thiophenes are tolerated in ADIMET with simple catalyst systems, the thiophene nucleus has to be relatively far away from the reacting propyne group (Scheme 8); 2-propynylthiophene 33 did not undergo ADIMET with simple catalyst systems (Scheme 9). Fu¨rstner [14] reported a highly active catalyst system that was formed from Cummins’ tris(amide) [Mo(NHR)3] [17] and dichloromethane in situ. According to the authors, this is the most active and heteroatom-tolerant catalyst available to date. Attempts to dimerize 33 with Fu¨rstner’s catalyst failed (Scheme 9), although co-metathesis with a second alkyne proceeded smoothly. Weiss [18] reacted 2,5-dipropynylthiophene 35 with 11, whereby small thiophenyleneethynylene oligomers 36 formed in moderate yields (Scheme 10).
Scheme 9
Scheme 10
7.2.3
Cycles
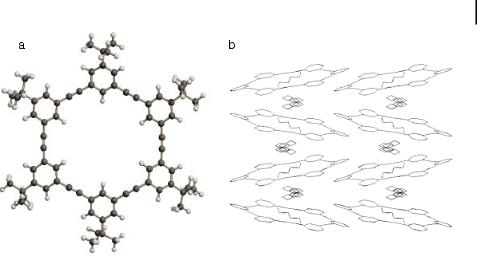
Having demonstrated that the dimerization of propynylated substrates works well, it was important to explore ring-forming metathesis (RCM) [14]. The first experiments utilizing 1,2-dipropynylbenzene 37 were unsuccessful, and led to the isolation of unreacted starting material along with a small amount of an infusible and intractable dark solid. Perhaps the second alkyne group in 37 chelates the molybdenum center and prevents metathesis activity. More successfully, subjecting 1,3-dipropynyl-5-tert-butylbenzene 39 to RCM conditions provided the cyclohexameric phenyleneethynylene 40 in an isolated yield of 8 % after repeated chromatography. According to mass spectrometry, the heptameric and octameric cycles are also formed, albeit in much lower yields. The other identified product of this reaction is a poly(metaphenyleneethynylene) of relatively low molecular weight [19]. To confirm the structure of 40, Adams, Bunz, and Herrmann performed a single-crystal X-ray
224 7 The ADIMET Reaction: Synthesis and Properties of Poly(dialkylparaphenyleneethynylene)s
Scheme 11
structure analysis [19]. The geometry of the ring is hexagonal, although the packing of the cycles is of more interest. Moore has recently described a packing motif of a hexaphenolic phenyleneethynylene cycle. This macrocycle forms extended channels, and the rings are packed in a coplanar fashion [20] on top of one another. In the simpler hydrocarbon system
7.2 Syntheses 225
Fig. 1. (a) Ball-and-stick plot of 40, (b) packing diagram of 40. The tertbutyl groups and the hydrogens are omitted for clarity. The squares indicate highly disordered solvent, probably hexanes.
40, a related packing motif is observed, but stacks of the rings that form channels are mutually arranged in a herringbone pattern. This would be expected for large aromatic molecules where only van der Waals and electrostatic forces play a role.
To explore other ring-forming metatheses, the trimeric precursor 41 was subjected to ADIMET, which produced a small percentage of the cycle 42 in addition to large amounts of a weakly fluorescent but not very soluble polymer. The substrate that proved most ideal for ring-forming ADIMET was 43, which, upon metathesis, furnished the trimeric cycle 44a ðn ¼ 1Þ in 14 % and the tetrameric cycle 44b ðn ¼ 2Þ in 18 % isolated yield after chromatography and crystallization. While larger ring sizes could be detected by mass spectrometry, it was not possible to isolate these rings by column chromatography [21].
7.2.4
Alkyne-Bridged Polymers by ADIMET
Conjugated polymers are organic semiconductors, and as such they are of great importance in the fabrication of organic semiconductor devices. These devices include, but are not restricted to, light-emitting diodes (LEDs), thin-film transistors, photovoltaic cells, and plastic lasers. The most important polymers in this field are the PPVs, despite their moderate stability, delicate processing requirements, and less than ideal photophysical properties [22]. The dehydrogenated congeners of the PPVs, the PPEs (12), show considerably higher stability, are more easily processible, and have superior photophysical properties in solution compared to those of the PPVs [1]. The PPEs have attracted much less attention in device fabrication, even though they have been popularized by Swager [23] as sensory materials and by Tour [24] as ‘‘molecular wires’’.
The classic synthesis of PPEs is the Pd/Cu-catalyzed coupling of diethynylbenzenes 45 with diiodoor dibromobenzenes 46 [1]. This coupling reaction is widely applicable, quite

226 7 The ADIMET Reaction: Synthesis and Properties of Poly(dialkylparaphenyleneethynylene)s
Scheme 12
general in scope, and was first utilized by Giesa and Schulz to prepare soluble PPEs [25]. However, the method is not without its problems, and the polymers 47 contain numerous defects, including diyne groupings. This is due to oxidative dimerization (by traces of oxygen or other oxidants in the reaction mixture) of the bis(alkyne) component. In addition, the endgroups of Pd-made PPEs are not well defined because of dehalogenation or phosphonium salt formation [26]. The reaction is a classic polycondensation, and is quite sensitive to imbalances in the stoichiometry. Furthermore, the degree of polymerization of the Pd-made PPEs is not very high (Pn < 50 repeating units) [1] unless acceptor-substituted dihalides are utilized [27]. The best polymers made by this method were described by Swager [28], who reacted diiodo aromatic coupling partners and a Pd(0) catalyst to minimize side reactions.
Because of the problems associated with Pd-catalyzed polycondensations, it became necessary to develop an independent, simple synthetic access that was not based on the Pd methodology. Alkyne metathesis was deemed appropriate, and with optimization of the simple ‘‘shake and bake’’ catalysts it was expected that it would be well-suited for forming PPEs [29]. The high yields obtained in the dimerization reactions of propynylated arenes (Schemes 7 and 8) strongly supported this notion, and from the work of Bunz, Mu¨llen, and Weiss it was clear that PPEs 12 could be made by ADIMET, at least if Schrock’s tungsten carbyne complex 11 was employed [13]. A reaction mixture containing dihexyldipropynylbenzene 10a and the ‘‘shake and bake’’ catalyst Mo(CO)6/4-chlorophenol became strongly fluorescent after approximately 1 h at a reaction temperature of 130 C, suggesting that productive metathesis had commenced (Scheme 13).
Scheme 13
Work-up furnished dihexyl-PPE 12a in an almost quantitative yield with a degree of polymerization (Pn) of approximately 100. It was noticed that 12a was only sparingly soluble in hot 1,2-dichlorobenzene. Dodecyl-, 2-ethylhexyl-, and bis(3,8-dimethyloctyl)dipropynylben-
|
|
|
|
|
|
7.2 Syntheses |
227 |
|
Tab. 1. Substituent pattern and molecular weights for representatitve PPEs 12. |
|
|
|
|||||
|
|
|
||||||
|
|
|
|
|
|
|
|
|
Substituent |
Hexyl a |
Hexyl a |
Hexyl a |
2-Ethylbutyl |
2-Ethylhexyl |
Dodecyl c |
||
|
|
|
|
e |
d |
|
|
|
|
|
|
|
|
|
|
|
|
Temp. ( C) |
120 |
170 |
150 |
150 |
150 |
150 |
|
|
Co-catalyst |
4-Cl-phenol |
4-Cl-phenol |
4-CF3-phenol |
4-CF3-phenol |
4-CF3-phenol |
4-Cl-phenol |
||
Yield |
70 |
76 |
99 |
80 |
99 |
96 |
|
|
Pn (GPC) |
22 |
10 |
94 |
insoluble |
510 |
770 |
|
|
|
|
|
|
|
|
|
|
|
zenes 10c,d,f were prepared by standard Pd-catalyzed reactions to improve the solubility and molecular weight. The ADIMET of 10c,d,f with the Mo(CO)6/4-chlorophenol mixture gave polymers 12c,d,f of higher molecular weight. The samples showed a degree of polymerization in the range Pn ¼ 200–700 [30, 31], as estimated by gel permeation chromatography (GPC). In rare cases, the Pn values exceeded 1000 repeat units. The degree of polymerization increases with the scale of the reaction and the purity of the monomers. An interesting point is the temperature window in which these reactions are successful. At 120 C, these polymerizations give low molecular weight oligomers, as is also the case above 160 C. From the data collected in South Carolina, the optimum conditions for ADIMET with Mori catalysts are 135–150 C, an excess of phenol, and a small amount of solvent. 4-Chlorophenol is not the only potent co-catalyst; 4-(trifluoromethyl)phenol/Mo(CO)6 is likewise e ective in promoting ADIMET.
The PPEs are isolated by precipitation from methanol after washing with acid and base to remove Mo-containing residues. The polymers are obtained as light-yellow to bright-yellow powders (branched side chains give paler polymers), flakes, or rubbery fibrous materials depending upon the nature of the side chain and the degree of polymerization. After 16 h, ADIMET gives materials of substantial molecular weight, and the reaction is finished after approximately 24–30 h, beyond which no increase in molecular weight is observed [31]. After 30 h, the concentration of reactive end-groups is very low, and it may be that the catalytic system slowly decomposes under these conditions. The reaction can be scaled-up; 10 g of PPE was prepared in a 250 mL Schlenk flask, suggesting that industrial scale synthesis of PPEs will be facile.
The 13C NMR spectra of dialkyl-PPEs (12) feature three signals in the aromatic region and one signal due to the alkyne units. No other resonances are detected in the region d ¼ 80– 200, excluding cross-linking by formation of networked PPV-type structures.
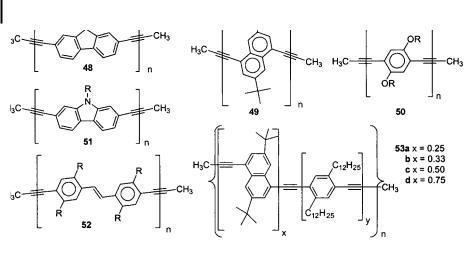
The synthetic utility of the ADIMET process with simple catalyst systems would be restricted if only PPE (albeit very pure and in high yields) could be made. Fortunately, this is not the case, and a series of di erent propynylated aromatics underwent ADIMET to give the polymers 48, 49, and 51–53 in good to excellent yields with respectable Pn values [32–35]. Most of these polymers were not known (49, 52, 53) or had never been appropriately characterized (48, 51). The PPVEs 52 are hybrids of PPEs and PPVs that have not been described previously. They may combine the stability of the PPEs with the exciting properties of the PPVs. Utilizing a series of dipropynylated stilbene derivatives accessible by a Horner–Wittig approach, alkyl-substituted PPVEs 52 of high molecular weight (Pn up to 400 aryleneethynylene units) and purity were obtained by ADIMET as stable, deep-yellow
228 7 The ADIMET Reaction: Synthesis and Properties of Poly(dialkylparaphenyleneethynylene)s
Scheme 14
or greenish emitting materials. PPVEs are of interest as active materials in organic lightemitting diodes [16].
Heteroatoms are tolerated in these polymerization reactions as long as they are spatially separated from the reacting propyne groups. An excellent example is the poly(carbazyleneethynylene) 51, which is made by ADIMET from N-alkyldipropynylcarbazoles [32]. The presence of oxygen functionalities is likewise tolerated, but the formation of dialkoxy-PPE 50 gives polymers of relatively low molecular weight, even though it proceeds in high yields. Polymer 50 is only formed when the active co-catalyst 4-(trifluoromethyl)phenol is employed. ADIMET allows the synthesis of copolymers, as exemplified by the formation of poly(naphthyleneethynylene)/PPE hybrid 53 when dipropynyldialkylbenzenes 10 and dipropynyldi-tert-butylnaphthalene are co-metathesized. Typically, Pn values of 50– 100 are achieved, and the polymers are formed in high yields. The copolymers are, like the poly(fluorenyleneethynylene)s 48, strongly solid-state blue-fluorescent emitting, and have been utilized in the fabrication of PPE-based OLEDs (vide infra). ADIMET-made poly(dialkylfluorenyleneethynylene)s 48 are now commercially available and are marketed by Aldrich.
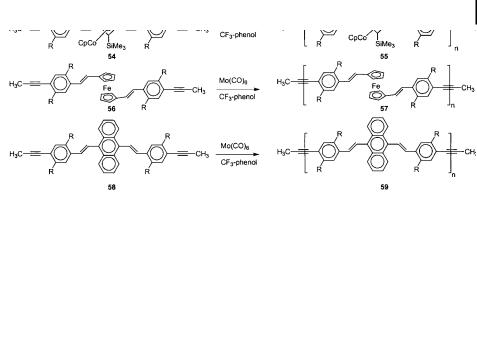
ADIMET is not restricted to the synthesis of simple poly(aryleneethynylene)s. If somewhat more complex monomers (54, 56, 58) are employed, organometallic and arylenevinylenecontaining modules can be embedded into the polymer backbone. The introduction of electroactive and other modules of interest is possible whenever propynylbenzene groups can be attached to the target core. The polymers 55, 57, 59 are stable under the reaction conditions, and the c[aAacaAacaBac]n sequence is not disturbed according to 13C NMR spectroscopy. That is to say, no scrambling of the arene units is observed under ADIMET conditions. By the same token, it was not possible to degrade 12c by the Mori catalysts in the presence of an excess of either dodecyne or diphenylacetylene (Scheme 16).
Overall, the most di cult part of the ADIMET process is the synthesis of the monomers. Monomer synthesis can be a challenge, considering that the necessary aromatic diiodides
7.3 Reactivities of PPEs 229
Scheme 15
Scheme 16
are often either not described in the literature or are di cult to prepare. Given the absence of chelating heteroatoms, most monomers bearing two propyne groups will be polymerizable by ADIMET with simple catalyst systems to give high molecular weight polymers in good yields and with high purity.
7.3
Reactivities of PPEs
The determination of the absolute molecular weights of rigid rods by GPC is problematic, because molecular weight standards for GPC are almost all (for an exception, see ref. [36]) based on polystyrene. Polystyrene is coiled and much more flexible than PPE. PPEs behave like tough rods with a persistence length of several tens of nm, as reported by Cotts and Swager [37]. Numerical values of by how much GPC overestimates the molecular weight of PPEs are di cult to determine, but Tour reported that the GPC values for phenyleneethynylene oligomers overestimate their real molecular weights by roughly a factor of two [38]. An interesting situation would arise if the PPEs could be transformed from rigid-rod polymers into flexible coils. Thus, a rigid rod could be compared to a flexible coil with the same polydispersity and the same molecular weight. PPEs should be uniquely suited for this purpose because catalytic hydrogenation of their triple bonds should lead to a coiled polymer with only a slightly increased molecular weight. The hydrogenated PPE would be an isomeric polystyrene 60 [39], in which the polymer connection is made through the 1,4- positions of the benzene ring instead of the benzene units substituting a polyethylene chain.

2307 The ADIMET Reaction: Synthesis and Properties of Poly(dialkylparaphenyleneethynylene)s
The polymer 60 is expected to be coiled and thus better amenable to reliable molecular weight determination by GPC using polystyrene standards.
Attempts to reduce PPEs 12 under moderate pressures (2–3 bar H2) at temperatures of around 70–80 C were unsuccessful. PPEs seem to be very stable chemically, and only when
the reduction was performed in an autoclave under drastic conditions successful hydrogenation was observed. Hydrogen pressures of 300–500 bar at temperatures of around 300 C are necessary for complete reduction of the PPE to the hydrogenated polymer 60 [40]. The yield of 60 is high; the aromatic rings are not a ected under these conditions and side products are not detected in the colorless materials formed. Attempts to use shorter reaction times, lower temperatures, or lower hydrogen pressures failed, and partially reacted material of undefined structure resulted.
Scheme 17
The hydrogenation of several low to medium molecular weight PPEs showed that the molecular weights of the PPEs and their hydrogenated congeners 60 can di er by a factor ranging from 1.1 to 1.6 depending upon the side chain. Further experiments are necessary to evaluate the relationship between the molecular weights of the rods and the corresponding coils.
Generally, the reactivity of PPEs is not well established. Weder has recently reported an interesting means of functionalizing PPEs via an organometallic route. Thus, treatment of PPE 61 with the styrene complex of platinum dichloride (62) furnished the polymeric PPE complexes 63, in which the content of the organometallic units could be controlled by the amount of Pt complex added. The labile styrene ligand in the platinated polymer 63 may be removed at a later stage to give three-dimensionally conjugated organometallic PPEheterostructures [41].
PPEs should o er a potent platform for polymer-analogous reactions, and a family of new materials should be accessible by either complexation or cycloaddition via their bridging triple bonds. A di culty is the relatively low reactivity of the alkyne units in PPEs. Steric shielding by the adjacent alkyl or alkoxy groups, as well as electronic e ects, reduce the re-

7.4 Solid-State Structures and Liquid-Crystalline Properties of the PPEs 231
Scheme 18
activity of the alkynes in the highly conjugated polymer and present formidable obstacles to the exploitation of this concept.
7.4
Solid-State Structures and Liquid-Crystalline Properties of the PPEs
PPEs are formally linear and rigid molecular rods with a high molecular anisotropy. As a result, PPEs have a large aspect ratio (aspect ¼ length/width). In the early contributions that dealt with the synthesis of dialkoxy-PPEs, it was claimed that PPEs were liquid crystalline [1]. However, no convincing proof was obtained. Optical micrographs showed shear-induced birefringence, but no identifiable liquid-crystalline textures pointed to a smectic or nematic phase [42]. In addition, the dialkoxy-PPEs are not thermally robust and they do not generally give a stable isotropic melt but decompose at temperatures above 120 C. Swager, Weder, and Wrighton [43], and Le Moigne [42] observed the lamellar packing of the PPEs by powder di raction analysis, which provided evidence of a smectic ordering in these materials. In 1997, Weder [44] demonstrated that dialkoxy-PPEs form lyotropic-nematic liquid-crystalline phases in highly concentrated solutions in 1,2-dichlorobenzene. However, there have been no reported cases of thermotropic dialkoxy-PPEs in the literature.
7.4.1
Organometallic Poly(aryleneethynylene)s
The first reported thermotropic and lyotropic liquid-crystalline poly(aryleneethynylene)s were not parent PPEs, but organometallic derivatives 65 made by the Pd-catalyzed condensation of Vollhardt’s diethynyl 64 with diiodobenzenes 46 [45]. The polymers were isolated in good to excellent yields as amorphous tan or red powders with moderate Pn values of
Scheme 19
232 7 The ADIMET Reaction: Synthesis and Properties of Poly(dialkylparaphenyleneethynylene)s
Tab. 2. Substituent pattern, yield, molecular weight, and polydispersities of 65a–c.
65 |
R1 |
R3 |
Yield (%) |
Mn (103) |
Pn |
Mw=Mn |
a |
SiMe3 |
Hexyl |
74 |
5.1 |
18 |
2.6 |
b |
SiMe3 |
Dodecyl |
79 |
4.9 |
12 |
4.1 |
c |
SiMe3 |
|
80 |
21 |
29 |
3.0 |
|
|
|
|
|
|
|
12–30 repeating units. The thermal and phase behaviors of 65a and 65c were investigated in detail. Both 65a and 65c form lyotropic nematic phases, as was evident from polarizing microscopy and powder di raction analyses.
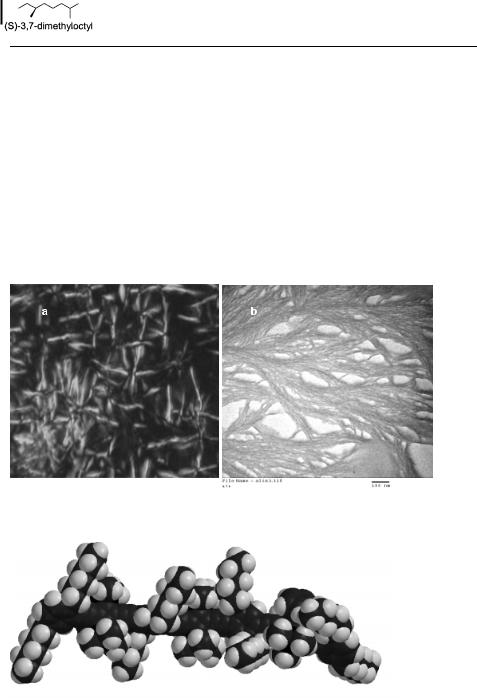
However, only 65a exhibits thermotropic nematic behavior if amorphous thin films are heated to 155 C, at which a Schlieren texture develops. The material decomposes above
Fig. 2. a) Polarizing micrograph of 65c. b) Transmission electron micrograph of 65a.
Fig. 3. Single chain geometry of a segment of the organometallic PPE derivative 65a. Planarization of the backbone in these materials is not favorable due to the added-on cyclopentadienylcobalt fragments that bulk up the main chain.
