
Astruc D. - Modern arene chemistry (2002)(en)
.pdf
|
|
|
References |
293 |
|
Soc. 1984, 106, 1132–1133; b) R. P. |
|
|
|
|
33 |
a) K. H. Do¨tz, D. Grotjahn, K. Harms, |
||
|
Hsung, W. D. Wulff, C. A. Challener, |
|
Angew. Chem. Int. Ed. Engl. 1989, 28, |
|
|
Synthesis 1996, 773–789. |
|
1384–1386; Angew. Chem. 1989, 101, |
|
18 |
J. Barluenga, F. Aznar, I. Gutie´rrez, |
|
1425–1427; b) D. B. Grotjahn, K. H. |
|
|
A. Martı´n, S. Garcı´a-Granda, M. A. |
|
Do¨tz, Synlett 1991, 381–390; c) D. B. |
|
|
Llorca-Baragan˜o, J. Am. Chem. Soc. |
|
Grotjahn, F. E. K. Kroll, T. Scha¨fer, K. |
|
|
2000, 122, 1314–1324. |
|
Harms, K. H. Do¨tz, Organometallics 1992, |
|
19 |
J. S. McCallum, F. A. Kunng, S. R. |
|
11, 298–310. |
|
|
Gilbertson, W. D. Wulff, |
34 |
a) J. Barluenga, L. A. Lo´pez, S. |
|
|
Organometallics 1988, 7, 2346–2360. |
|
Martı´nez, M. Toma´s, J. Org. Chem. 1998, |
|
20 |
A. Yamashita, Tetrahedron Lett. 1986, 27, |
|
63, 7588–7589; b) J. Barluenga, L. A. |
|
|
5915–5918. |
|
Lo´pez, S. Martinez, M. Toma´s, |
|
21 |
K. S. Chan, G. A. Peterson, T. A. |
|
Tetrahedron 2000, 56, 4967–4975. |
|
|
Brandvold, K. L. Faron, C. A. Chal- |
35 |
a) M. E. Bos, W. D. Wulff, R. A. Miller, |
|
|
lener, C. Hyldahl, W. D. Wulff, J. |
|
S. Chamberlin, T. A. Brandvold, J. Am. |
|
|
Organomet. Chem. 1987, 334, 9–56. |
|
Chem. Soc. 1991, 113, 9293–9319; b) V. P. |
|
22 |
a) E. O. Fischer, A. Maasbo¨l, Angew. |
|
Liptak, W. D. Wulff, Tetrahedron 2000, |
|
|
Chem. Int. Ed. Engl. 1964, 3, 580; Angew. |
|
56, 10229–10247. |
|
|
Chem. 1964, 76, 645; b) E. O. Fischer, A. |
36 |
a) K. H. Do¨tz, R. Dietz, Chem. Ber. 1978, |
|
|
Maasbo¨l, Chem. Ber. 1967, 100, 2445– |
|
111, 2517–2526; b) J. Pfeiffer, M. |
|
|
2456. |
|
Nieger, K. H. Do¨tz, Chem. Eur. J. 1998, 4, |
|
23 |
E. O. Fischer, R. Aumann, Chem. Ber. |
|
1843–1851. |
|
|
1968, 101, 954–962. |
37 |
a) K. H. Do¨tz, R. Dietz, Chem. Ber. 1977, |
|
24 |
L. S. Hegedus, M. A. McGuire, L. M. |
|
110, 1555–1563; b) W. D. Wulff, K.-S. |
|
|
Schultze, Org. Synth. 1987, 65, 216–219. |
|
Chan, J. Org. Chem. 1984, 49, 2293–2295; |
|
25 |
a) T. R. Hoye, K. Chen, J. R. Vyvyan, |
|
c) B. A. Anderson, J. Bao, T. A. |
|
|
Organometallics 1993, 12, 2806–2809; b) |
|
Brandvold, C. A. Challener, W. D. |
|
|
Q.-H. Zheng, J. Su, Synth. Commun. |
|
Wulff, Y.-C. Xu, A. L. Rheingold, J. Am. |
|
|
2000, 30, 177–185. |
|
Chem. Soc. 1993, 115, 10671–10687. |
|
26 |
E. O. Fischer, T. Selmayr, F. R. Kreissl, |
38 |
a) K. H. Do¨tz, R. Ehlenz, D. Paetsch, |
|
|
Chem. Ber. 1977, 110, 2947–2955. |
|
Angew. Chem. Int. Ed. Engl. 1997, 36, |
|
27 |
a) M. F. Semmelhack, S. R. Lee, |
|
2376–2378; Angew. Chem. 1997, 109, |
|
|
Organometallics 1987, 6, 1839–1844; |
|
2473–2475; b) K. H. Do¨tz, R. Ehlenz, |
|
|
b) R. Imwinkelried, L. S. Hegedus, |
|
Chem. Eur. J. 1997, 3, 1751–1756; d) M. R. |
|
|
Organometallics 1988, 7, 702–706; c) M. A. |
|
Hallet, J. E. Painter, P. Quayle, D. |
|
|
Schwindt, T. Lejon, L. S. Hegedus, |
|
Ricketts, P. Patel, Tetrahedron Lett. 1998, |
|
|
Organometallics 1990, 9, 2814–2819. |
|
39, 2851–2852; e) K. H. Do¨tz, F. Otto, M. |
|
28 |
a) M. L. Waters, T. A. Brandvold, L. |
|
Nieger, J. Organomet. Chem. 2001, 621, |
|
|
Isaacs, W. D. Wulff, A. L. Rheingold, |
|
77–88. |
|
|
Organometallics 1998, 17, 4298–4308; b) |
39 |
a) K. H. Do¨tz, M. Popall, G. Mu¨ller, K. |
|
|
S. R. Pulley, S. Sen, A. Vorogushin, E. |
|
Ackermann, Angew. Chem. Int. Ed. Engl. |
|
|
Swanson, Org. Lett. 1999, 1, 1721–1723. |
|
1986, 25, 911–912; Angew. Chem. 1986, 98, |
|
29 |
M. Yamashita, T. Ohishi, Bull. Chem. |
|
909–910; b) K. H. Do¨tz, M. Popall, G. |
|
|
Soc. Jpn. 1993, 66, 1187–1190. |
|
Mu¨ller, K. Ackermann, J. Organomet. |
|
30 |
a) K. H. Do¨tz, C. Stinner, unpublished |
|
Chem. 1990, 383, 93–111. |
|
|
results; b) M. F. Gross, M. G. Finn, |
40 |
a) J. Christoffers, K. H. Do¨tz, J. Chem. |
|
|
unpublished results as cited in footnote |
|
Soc., Chem. Commun. 1993, 1811–1812; b) |
|
|
[41b] in ref. [31]. |
|
J. Christoffers, K. H. Do¨tz, Chem. Ber. |
|
31 |
M. F. Gross, M. G. Finn, J. Am. Chem. |
|
1995, 128, 157–161. |
|
|
Soc. 1994, 116, 10921–10933. |
41 |
K. H. Do¨tz, J. Gla¨nzer, J. Chem. Soc., |
|
32 |
a) A. Yamashita, A. Toy, N. B. Ghazal, |
|
Chem. Commun. 1993, 1036–1037. |
|
|
C. R. Muchmore, J. Org. Chem. 1989, 54, |
42 |
K. H. Do¨tz, S. Klapdohr, unpublished |
|
|
4481–4483; b) K. H. Do¨tz, V. Leue, J. |
|
results. |
|
|
Organomet. Chem. 1991, 407, 337–351. |
43 |
a) R. Dietz, K. H. Do¨tz, D. Neugebauer, |
|

294 8 The Chromium-Templated Carbene Benzannulation Approach to Densely Functionalized Arenes
|
Nouv. J. Chim. 1977, 2, 59–61; b) J. W. |
|
Keller, T. Sato, E. J. Spiess, W. Wulff, |
|
Herndon, A. Hayford, Organometallics |
|
A. Zask, Tetrahedron 1985, 41, 5803– |
|
1995, 14, 1556–1558; c) J. W. Herndon, |
|
5812. |
|
H. Wang, J. Org. Chem. 1998, 63, 4562– |
55 |
a) S. Chamberlin, M. L. Waters, W. D. |
|
4563; d) Y. Zhang, J. W. Herndon, |
|
Wulff, J. Am. Chem. Soc. 1994, 116, |
|
Tetrahedron 2000, 56, 2175–2182. |
|
3113–3114; b) M. F. Semmelhack, S. Ho, |
44 |
K. H. Do¨tz, B. Fu¨gen-Ko¨ster, Chem. Ber. |
|
D. Cohen, M. Steigerwald, M. C. Lee, G. |
|
1980, 113, 1449–1457. |
|
Lee, A. M. Gilbert, W. D. Wulff, R. G. |
45 |
a) K. H. Do¨tz, I. Pruskil, Chem. Ber. |
|
Ball, J. Am. Chem. Soc. 1994, 116, 7108– |
|
1978, 111, 2059–2063; b) K. H. Do¨tz, D. |
|
7122. |
|
Neugebauer, Angew. Chem. Int. Ed. Engl. |
56 |
G. Schmalz, S. Siegel, in Transition |
|
1978, 17, 851–852; Angew. Chem. 1978, 90, |
|
Metals for Organic Synthesis, Vol. 1 (Ed.: M. |
|
898–899. |
|
Beller, C. Bolm), Wiley-VCH, Weinheim, |
46 |
See, for example: J. King, P. Quayle, J. F. |
|
1998, pp. 550–559. |
|
Malone, Tetrahedron Lett. 1990, 31, 5221– |
57 |
a) R. P. Hsung, W. D. Wulff, A. L. |
|
5224. |
|
Rheingold, J. Am. Chem. Soc. 1994, 116, |
47 |
M. F. Semmelhack, N. Jeong, G. R. Lee, |
|
6449–6450; b) R. L. Beddoes, J. D. King, |
|
Tetrahedron Lett. 1990, 31, 609–610. |
|
P. Quayle, Tetrahedron Lett. 1995, 36, |
48 |
O. Kretschik, M. Nieger, K. H. Do¨tz, |
|
3027–3028; c) K. H. Do¨tz, C. Stinner, M. |
|
Organometallics 1996, 15, 3625–3629. |
|
Nieger, J. Chem. Soc., Chem. Commun. |
49 |
a) D. M. Gordon, S. J. Danishefsky, |
|
1995, 2535–2536; d) K. H. Do¨tz, C. |
|
G. K. Schulte, J. Org. Chem. 1992, 57, |
|
Stinner, Tetrahedron: Asymmetry 1997, 8, |
|
7052–7055; b) R. Zimmer, H.-U. |
|
1751–1765; e) R. P. Hsung, W. D. Wulff, |
|
Reissig, J. Prakt. Chem. 1998, 340, 755– |
|
S. Chamberlin, Y. Liu, R.-Y. Liu, H. |
|
756. |
|
Wang, J. F. Quinn, S. L. B. Wang, A. L. |
50 |
K. H. Do¨tz, I. Pruskil, Chem. Ber. 1980, |
|
Rheingold, Synthesis 2000, 200–220. |
|
113, 2876–2883. |
58 |
a) J. P. A. Harrity, W. J. Kerr, D. |
51 |
a) C.-S. Chan, C. C. Mak, K.-S. Chan, |
|
Middlemiss, Tetrahedron Lett. 1993, 34, |
|
Tetrahedron Lett. 1993, 34, 5125–5126; b) |
|
2995–2998; b) J. P. A. Harrity, W. J. |
|
C.-S. Chan, A. K.-S. Tse, K. S. Chan, J. |
|
Kerr, D. Middlemiss, Tetrahedron 1993, |
|
Org. Chem. 1994, 59, 6084–6089; c) K. S. |
|
49, 5565–5576; c) Y. H. Choi, K. S. Rhee, |
|
Chan, H. Zhang, Synth. Commun. 1995, |
|
K. S. Kim, G. C. Shin, S. C. Chin, |
|
25, 635–639; d) S. R. Pulley, J. P. Carey, |
|
Tetrahedron Lett. 1995, 36, 1871–1874. |
|
J. Org. Chem. 1998, 63, 5275–5279; e) D. |
59 |
a) E. O. Fischer, H. Fischer, Chem. Ber. |
|
Paetsch, K. H. Do¨tz, Tetrahedron Lett. |
|
1974, 107, 657–672; b) E. O. Fischer, H. |
|
1999, 40, 487–488. |
|
Fischer, Chem. Ber. 1974, 107, 673–679; |
52 |
a) K. H. Do¨tz, A. Tiriliomis, K. Harms, |
|
c) J. R. Knorr, T. L. Brown, Organometal- |
|
M. Regitz, U. Annen, Angew. Chem. Int. |
|
lics 1994, 13, 2178–2185; d) L. S. Hegedus, |
|
Ed. Engl. 1988, 27, 713–715; Angew. Chem. |
|
Tetrahedron 1997, 53, 4105–4128. |
|
1988, 100, 725–727; b) K. H. Do¨tz, A. |
60 |
a) C. A. Merlic, D. Xu, J. Am. Chem. Soc. |
|
Tiriliomis, K. Harms, J. Chem. Soc., |
|
1991, 113, 7418–7420; b) C. A. Merlic, |
|
Chem. Commun. 1989, 788–790; c) K. H. |
|
E. E. Burns, D. Xu, S. Y. Chen, J. Am. |
|
Do¨tz, A. Tiriliomis, K. Harms, |
|
Chem. Soc. 1992, 114, 8722–8724; c) C. A. |
|
Tetrahedron 1993, 49, 5577–5597. |
|
Merlic, E. E. Burns, Tetrahedron Lett. |
53 |
a) W. D. Wulff, P.-C. Tang, J. S. |
|
1993, 34, 5401–5404; d) C. A. Merlic, |
|
McCallum, J. Am. Chem. Soc. 1981, 103, |
|
W. M. Roberts, Tetrahedron Lett. 1993, 34, |
|
7677–7678; b) K. H. Do¨tz, J. Mu¨hle- |
|
7379–7382; e) C. A. Merlic, D. Xu, B. G. |
|
meier, U. Schubert, O. Orama, J. |
|
Gladstone, J. Org. Chem. 1993, 58, 538– |
|
Organomet. Chem. 1983, 247, 187–201; c) |
|
545; f) J. Barluenga, F. Aznar, M. A. |
|
A. Yamashita, A. Toy, Tetrahedron Lett. |
|
Palomero, S. Barluenga, Org. Lett. 1999, |
|
1986, 27, 3471–3474. |
|
1, 541–543; g) J. Barluenga, F. Aznar, |
54 |
a) M. F. Semmelhack, J. J. Bozell, |
|
M. A. Palomero, Angew. Chem. Int. Ed. |
|
Tetrahedron Lett. 1982, 23, 2931–2934; b) |
|
Engl. 2000, 39, 4346–4348; Angew. Chem. |
|
M. F. Semmelhack, J. J. Bozell, L. |
|
2000, 112, 4514–4516; h) J. Barluenga, |
|
|
|
References |
295 |
|
|
|
|
|
|
F. Aznar, M. A. Palomero, Chem. Eur. J. |
|
615; b) J. Schulz, F. Vo¨gtle, Top. Curr. |
|
|
2001, 7, 5318–5324. |
|
Chem. 1994, 72, 42–89. |
|
61 |
a) B. Weyershausen, K. H. Do¨tz, Eur. J. |
70 |
A. de Meijere, J. Ho¨fer, unpublished |
|
|
Org. Chem. 1998, 1739–1742; b) B. |
|
results, as cited in ref. [8c]. |
|
|
Weyershausen, K. H. Do¨tz, Synlett 1999, |
71 |
A. Longen, M. Nieger, K. Airola, K. H. |
|
|
231–233. |
|
Do¨tz, Organometallics 1998, 17, 1538– |
|
62 |
a) S. Chamberlin, W. D. Wulff, B. Bax, |
|
1545. |
|
|
Tetrahedron 1993, 49, 5531–5547; for a |
72 |
J. Schulz, M. Nieger, F. Vo¨gtle, Chem. |
|
|
recent example of in situ protection, see: |
|
Ber. 1991, 124, 2797–2810. |
|
|
b) W. H. Moser, L. Sun, J. C. Huffman, |
73 |
a) K. H. Do¨tz, A. Gerhardt, J. Organomet. |
|
|
Org. Lett. 2001, 3, 3389–3391. |
|
Chem. 1999, 578, 223–228; b) K. H. Do¨tz, |
|
63 |
a) A. M. Gilbert, W. D. Wulff, J. Am. |
|
S. Mittenzwey, Eur. J. Org. Chem. 2002, |
|
|
Chem. Soc. 1994, 116, 7449–7450; b) |
|
39–47. |
|
|
S. Chamberlin, W. D. Wulff, J. Am. |
74 |
a) H. Wang, W. D. Wulff, J. Am. Chem. |
|
|
Chem. Soc. 1992, 114, 10667–10669. |
|
Soc. 1998, 120, 10573–10574; b) H. Wang, |
|
64 |
a) M. W. Davies, C. N. Johnson, J. P. A. |
|
W. D. Wulff, A. L. Rheingold, J. Am. |
|
|
Harrity, Chem. Commun. 1999, 2107– |
|
Chem. Soc. 2000, 122, 9862–9863. |
|
|
2108; b) M. W. Davies, C. N. Johnson, |
75 |
a) R. Neidlein, P. J. Rosyk, W. Kramer, |
|
|
J. P. A. Harrity, J. Org. Chem. 2001, 66, |
|
H. Suschitzky, Synthesis 1991, 123–125; |
|
|
3525–3532. |
|
b) R. Neidlein, S. Gu¨rtler, C. Krieger, |
|
65 |
a) Y. F. Oprunenko, N. G. Akhmedov, |
|
Helv. Chim. Acta 1994, 77, 2303–2322; c) |
|
|
D. N. Roznyakovsky, Y. A. Ustynyuk, N. |
|
R. Neidlein, J. Teichmann, unpublished |
|
|
A. Ustynyuk, J. Organomet. Chem. 1999, |
|
results. |
|
|
583, 136–145; b) Y. Oprunenko, S. |
76 |
L. Quast, M. Nieger, K. H. Do¨tz, |
|
|
Malyugina, P. Nesterenko, D. Mityuk, |
|
Organometallics 2000, 19, 2179–2183. |
|
|
O. Malyshev, J. Organomet. Chem. 2000, |
77 |
a) J. M. Heerding, H. W. Moore, J. Org. |
|
|
597, 42–47; c) K. H. Do¨tz, N. Szesni, M. |
|
Chem. 1991, 56, 4048–4050; b) S. Koo, |
|
|
Nieger, K. Na¨ttinen, submitted for |
|
L. S. Liebeskind, J. Am. Chem. Soc. 1995, |
|
|
publication. |
|
117, 3389–3404. |
|
66 |
K. S. Chan, C. C. Mak, Tetrahedron 1994, |
78 |
a) M. Iwasaki, H. Matsuzaka, Y. Hiroe, |
|
|
50, 2003–2016. |
|
Y. Ishii, Y. Koyasu, M. Hidai, Chem. Lett. |
|
67 |
J. M. Timko, A. Yamashita, Org. Synth. |
|
1988, 1159–1162; b) M. Iwasaki, Y. Ishii, |
|
|
1993, 71, 72–76. |
|
M. Hidai, J. Organomet. Chem. 1991, 415, |
|
68 |
a) N. Hoa Tran Huy, P. Lefloch, J. |
|
435–442. |
|
|
Organomet. Chem. 1988, 344, 303–311; |
79 |
K. H. Do¨tz, M. Popall, Tetrahedron 1985, |
|
|
b) K. A. Parker, C. A. Coburn, J. Org. |
|
41, 5797–5802. |
|
|
Chem. 1991, 56, 1666–1668; c) J. Bao, W. |
80 |
H. C. Jahr, M. Nieger, K. H. Do¨tz, J. |
|
|
D. Wulff, M. J. Fumo, E. B. Grant, D. P. |
|
Organomet. Chem. 2002, 641, 185–194. |
|
|
Heller, M. C. Whitcomb, S.-M. Yeung, |
81 |
a) K. H. Do¨tz, S. Siemoneit, F. |
|
|
J. Am. Chem. Soc. 1996, 118, 2166–2181; |
|
Hohmann, M. Nieger, J. Organomet. |
|
|
d) J. Bao, W. D. Wulff, J. B. Dominy, |
|
Chem. 1997, 541, 285–290; b) F. Hoh- |
|
|
M. J. Fumo, E. B. Grant, A. C. Rob, |
|
mann, S. Siemoneit, M. Nieger, K. H. |
|
|
M. C. Whitcomb, S.-M. Yeung, R. L. |
|
Do¨tz, Chem. Eur. J. 1997, 3, 853–859. |
|
|
Ostrander, A. L. Rheingold, J. Am. |
82 |
a) A. Yamashita, T. A. Scahill, C. G. |
|
|
Chem. Soc. 1996, 118, 3392–3405; e) P. |
|
Chidester, Tetrahedron Lett. 1985, 26, |
|
|
Tomuschat, L. Kro¨ner, E. Steckhan, |
|
1159–1162; b) A. Yamashita, T. A. |
|
|
M. Nieger, K. H. Do¨tz, Chem. Eur. J. |
|
Scahill, A. Toy, Tetrahedron Lett. 1985, |
|
|
1999, 5, 700–707; f ) L. Fogel, R. P. |
|
26, 2969–2972; c) A. Yamashita, J. M. |
|
|
Hsung, W. D. Wulff, R. G. Sommer, A. |
|
Timko, W. Watt, Tetrahedron Lett. 1988, |
|
|
L. Rheingold, J. Am. Chem. Soc. 2001, |
|
29, 2513–2516; d) W. D. Wulff, J. S. |
|
|
123, 5580–5581. |
|
McCallum, F.-A. Kunng, J. Am. Chem. |
|
69 |
a) A. de Meijere, O. Reiser, M. Sto¨bbe, J. |
|
Soc. 1988, 110, 7419–7434; e) A. Yama- |
|
|
Kopf, G. Adiwidjaja, V. Sinnwell, S. I. |
|
shita, A. Toy, T. A. Scahill, J. Org. |
|
|
Khan, Acta Chem. Scand. 1988, A42, 611– |
|
Chem. 1989, 54, 3625–3634; f ) A. |
|

296 8 The Chromium-Templated Carbene Benzannulation Approach to Densely Functionalized Arenes
|
Yamashita, R. G. Schaub, M. K. Bach, |
90 |
For a review of synthetic strategies towards |
|
G. J. White, A. Toy, N. B. Ghazal, M. D. |
|
daunomycinone, see: P. J. Harrington, |
|
Burdick, J. R. Brashler, M. S. Holm, J. |
|
Transition Metals in Total Synthesis, Wiley, |
|
Med. Chem. 1990, 33, 775–781; g) K. H. |
|
New York, 1990, pp. 346–399. |
|
Do¨tz, J. Gla¨nzer, Z. Naturforsch. 1993, |
91 |
a) M. F. Semmelhack, J. J. Bozell, T. |
|
48b, 2969–2972. |
|
Sato, W. Wulff, E. Spiess, A. Zask, J. |
83 |
a) T. Leese, K. H. Do¨tz, Chem. Ber. 1996, |
|
Am. Chem. Soc. 1982, 104, 5850–5852; b) |
|
129, 623–631; b) K. H. Do¨tz, T. Leese, |
|
K. H. Do¨tz, M. Popall, G. Mu¨ller, J. |
|
Bull. Soc. Chim. Fr. 1997, 134, 503–515. |
|
Organomet. Chem. 1987, 334, 57–75; c) |
84 |
a) C. A. Merlic, A. Baur, C. C. Aldrich, |
|
D. Boger, I. C. Jacobson, J. Org. Chem. |
|
J. Am. Chem. Soc. 2000, 122, 7398–7399; |
|
1991, 56, 2115–2122; d) K. H. Do¨tz, W. A. |
|
b) W. E. Bauta, W. D. Wulff, S. F. |
|
Donaldson, W. Sturm, Synth. Commun. |
|
Pavkovic, E. J. Zaluzec, J. Org. Chem. |
|
2000, 30, 3775–3784; e) X. Xie, M. C. |
|
1989, 54, 3249–3252; c) M. Mori, K. |
|
Kozlowski, Org. Lett. 2001, 3, 2661– |
|
Kuriyama, N. Ochifuji, S. Watanuki, |
|
2663. |
|
Chem. Lett. 1995, 615–616. |
92 |
a) D. L. Boger, I. C. Jacobson, Tetra- |
85 |
K. S. Chan, W. D. Wulff, J. Am. Chem. |
|
hedron Lett. 1989, 30, 2037–2040; b) D. L. |
|
Soc. 1986, 108, 5229–5236. |
|
Boger, I. C. Jacobson, J. Org. Chem. |
86 |
G. A. Peterson, W. D. Wulff, Tetrahedron |
|
1990, 55, 1919–1928; c) D. L. Boger, O. |
|
Lett. 1997, 38, 5587–5590. |
|
Hu¨ter, K. Mbiya, M. Zhang, J. Am. |
87 |
W. Flitsch, J. Lauterwein, W. Micke, |
|
Chem. Soc. 1995, 117, 11839–11849. |
|
Tetrahedron Lett. 1989, 30, 1633–1636. |
93 |
a) J. Bao, V. Dragisich, S. Wenglowsky, |
88 |
a) K. H. Do¨tz, I. Pruskil, J. |
|
W. D. Wulff, J. Am. Chem. Soc. 1991, |
|
Mu¨hlemeier, Chem. Ber. 1982, 115, 1278– |
|
113, 9873–9875; b) J. Bao, W. D. Wulff, |
|
1285; b) K. H. Do¨tz, W. Kuhn, Angew. |
|
V. Dragisich, S. Wenglowsky, R. G. |
|
Chem. Int. Ed. Engl. 1983, 22, 732; Angew. |
|
Ball, J. Am. Chem. Soc. 1994, 116, 7616– |
|
Chem. 1983, 95, 750–751; Angew. Chem. |
|
7630. |
|
Suppl. 1983, 1045–1052. |
94 |
P. D. Woodgate, H. S. Sutherland, |
89 |
a) W. D. Wulff, P.-C. Tang, J. Am. Chem. |
|
C. E. F. Rickard, J. Organomet. Chem. |
|
Soc. 1984, 106, 434–436; b) W. D. Wulff, |
|
2001, 627, 206–220. |
|
P.-C. Tang, K.-S. Chan, J. S. McCallum, |
95 |
J. D. King, P. Quayle, Tetrahedron Lett. |
|
D. C. Yang, S. R. Gilbertson, Tetrahedron |
|
1991, 32, 7759–7762. |
|
1985, 41, 5813–5832; c) K. H. Do¨tz, M. |
96 |
a) C. A. Merlic, D. M. Meinnes, Y. You, |
|
Popall, Chem. Ber. 1988, 121, 665–672; d) |
|
Tetrahedron Lett. 1997, 38, 6787–6790; b) |
|
W. D. Wulff, Y.-C. Xu, J. Am. Chem. Soc. |
|
C. A. Merlic, Y. You, D. M. Innes, A. L. |
|
1988, 110, 2312–2314; e) J. Su, W. D. |
|
Zechman, M. M. Miller, Q. Deng, |
|
Wulff, R. G. Ball, J. Org. Chem. 1998, |
|
Tetrahedron 2001, 57, 5199–5212. |
|
63, 8440–8447; f ) W. D. Wulff, J. Su, |
97 |
A. V. Vorogushin, W. D. Wulff, |
|
P.-C. Tang, Y.-C. Xu, Synthesis 1999, 415– |
|
H.-J. Hansen, Org. Lett. 2001, 3, 2641– |
|
422. |
|
2644. |

297
9
Osmiumand Rhenium-Mediated Dearomatization Reactions with Arenes
Mark T. Valahovic, Joseph M. Keane, and W. Dean Harman
Abstract
The fragments fOs(NH3)5g2þ and fTpRe(CO)(L)g (where L ¼ 1-methylimidazole, pyridine, PMe3, or tert-butylisonitrile) form stable h2-coordinate complexes with a wide variety of arenes. The act of coordination greatly reduces the aromatic character of these ligands and, as a consequence, activates them towards various organic reactions. In particular, the addition of carbon-based electrophiles to arenes is notably enhanced relative to the free aromatic molecules. The resulting arenium intermediates are stabilized by metal backbonding to the point that they may be isolated and subsequently subjected to a variety of carbon-based nucleophiles. The overall vicinal difunctionalization of two ring carbons may be accomplished with excellent stereoand regiocontrol.
9.1
Introduction
Arenes are attractive building blocks for the synthesis of complex carbocyclic species. In addition to being inexpensive and available in a variety of substitution patterns, they possess ring structures composed entirely of unsaturated carbons. Because of this unsaturation, they have the potential for extensive functionalization. However, the realization of this potential requires synthetic methods that overcome the energetic barrier associated with aromatic stabilization.
Transition metals continue to be enticing reagents for the dearomatization of aromatic molecules [1]. Not only do they allow transformations to be performed on the dearomatized species at (sub)ambient temperatures, but they also serve to stabilize the reaction intermediates. This latter facet allows a much broader range of manipulations than those accessible through the typical electrophilic/nucleophilic aromatic substitution pathways.
During the past 40 years, two complementary general methodologies for transition metal based dearomatization have been developed. The fCr(CO)3g fragment [2, 3] and related cationic fragments (fMn(CO)3gþ [3–7], fCpRugþ [8, 9], and fCpFegþ [10]) are coordinated by aromatic molecules in a hexahapto fashion and have been shown to behave like electronwithdrawing groups. The coordinated arene is rendered electron-deficient and susceptible to nucleophilic attack. The resulting cyclohexadienyl anion is stabilized by the withdrawal of electron density from the metal fragment (Figure 1).
Modern Arene Chemistry. Edited by Didier Astruc
Copyright 8 2002 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim ISBN: 3-527-30489-4

298 9 Osmiumand Rhenium-Mediated Dearomatization Reactions with Arenes
Fig. 1. Transition metal dearomatization methodologies.
Alternatively, the fOs(NH3)5g2þ fragment (hereafter abbreviated as [Os]) and the electronically similar fTpRe(CO)(L)g fragment (Tp ¼ hydridotris(pyrazolyl)borate, L ¼ a strongly s-donating ligand) are coordinated by aromatic molecules in a dihapto fashion. Despite the 2þ charge on the Os and the p-acidic CO on the Re, these fragments are electron-rich and donate p-electron density into the p-system of the aromatic ligand, thereby increasing its nucleophilicity. Reactions of the bound aromatics with electrophiles result in cyclohexadienyl cations that are stabilized by the donation of electron density from the metal fragment. These intermediates can be either further elaborated or rearomatized, and removal of the metal fragment releases the modified organic molecule.
9.2
{Os(NH3)5}2B – The Pentaammineosmium(II) Fragment
9.2.1
Preparation of h2-Arene Complexes
The precursor to h2-complexes of pentaammineosmium(II), [Os(NH3)5(OTf )](OTf )2 (OTf ¼ trifluoromethanesulfonate), is commercially available from Aldrich and can be prepared in three steps from OsO4 (92 % overall yield) [11]. Thermally stable complexes of benzenes, naphthalenes, anisoles, anilines, and phenols can be prepared in yields >90 % by reducing this osmium(III) salt in an excess of the aromatic compound. Typically, the reductions are performed under a dinitrogen atmosphere using either Mgo in a DMA/DME mixture (DMA ¼ N,N-dimethylacetamide; DME ¼ 1,2-dimethoxyethane) or Zn/Hg in methanol. The h2-complexes are isolated by removing the reductant by filtration and precipitating the osmium salt from a solution in diethyl ether/dichloromethane. Excess ligand can be recovered from the resulting filtrate and recycled. Dihapto-coordinated arene complexes of [Os] are stable in solution under an inert atmosphere, and alkene complexes that result from dearomatization are stable to oxygen.
9.2.2
Binding Selectivity
Almost all h2-arene complexes of [Os] are amenable to both intrafacial (i.e., ring-walk) and interfacial (i.e., face-flip) isomerization mechanisms, which allow the metal to coordinate to the most thermodynamically favorable position (Figure 2). Aromatic molecules bearing a p- donor group (e.g., anisole, aniline, phenol) tend to place the metal across C5–C6 in order to

9.2 {Os(NH3)5}2þ --- The Pentaammineosmium(II) Fragment 299
Fig. 2. Binding selectivities of [Os]-arene complexes.
maintain linear conjugation of the donor group and the uncoordinated diene. (Coordination of the metal across C4–C5 sets up a cross-conjugated relationship between the donor group and the diene.) Naphthalene, having no electron donors, tends to place the metal across C3–C4 instead of C2–C3. In this way, the aromaticity of the unbound ring is maintained (Figure 2).
9.2.3
Hydrogenations
The coordination of an arene to [Os] serves to protect one double bond while rendering the remaining unsaturated carbons of the pendant ring more susceptible to hydrogenation than those of the corresponding free arene. While arene hydrogenations typically require harsh conditions and often lead to complete saturation, the partial hydrogenation of complexed benzene (1) proceeds in 15 h at 30 C under 1 atm. of H2 to give complexed cyclohexene (2) in 89 % yield (Figure 3) [12]. Likewise, complexed naphthalene (3) yields complexed 1,2- dihydronaphthalene (4), leaving the uncomplexed and therefore unactivated ring intact.
Steric arguments suggest that the addition of dihydrogen to [Os]-arenes should occur on the arene face opposite that of metal coordination. This theory is supported by the generation of 2-d4 upon the partial deuteration of 1. Furthermore, the hydrogenation of complexed anisole (5) under anhydrous conditions yields complexed 3-methoxycyclohexene (6), in which the methoxy group is syn to the metal. It was found, however, a trace of water in the reaction mixture results in the formation of complexed cyclohexenone (7), presumably from the attack of water on the dihydroanisole intermediate [13].

300 9 Osmiumand Rhenium-Mediated Dearomatization Reactions with Arenes
Fig. 3. Hydrogenations of [Os]-arenes.
9.2.4
Benzene and Alkylated Benzenes
The dearomatization of benzene requires that a resonance energy estimated at 36 kcal mol 1 be overcome [14]. For this reason, when benzene attacks an electrophile, the end result is typically a substitution product rather than an addition product. The initial cyclohexadienyl cation is unstable to deprotonation by even extremely poor bases, and it therefore loses a proton and returns to an aromatic state.
9.2.4.1 Benzene
Through a significant p-backbonding interaction, the coordination of benzene to [Os] (1) serves both to activate the arene toward the electrophilic addition of dimethoxymethane (Table 1, entries 1–4) or 3-penten-2-one (entry 5) and to stabilize the resulting benzenium intermediate 8. If manipulated at low temperature ( 40 C), 8 can be trapped with either a silyl ketene acetal (entries 1, 4, and 5), 2-trimethylsiloxypropene (entry 2), or phenyllithium (entry 3) to yield the substituted 1,4-cyclohexadiene complex 9 [15]. This species can be oxi-

9.2 {Os(NH3)5}2þ --- The Pentaammineosmium(II) Fragment 301
Tab. 1. Tandem additions to the benzene complex 1.
Entry |
E |
Nu |
Yield (%) |
1 |
|
|
82 |
2 |
23 |
3 |
16 |
4 |
31 |
5 |
27 |
dized to yield the dearomatized organic product 10. The nucleophile and electrophile add in a syn fashion, consistent with approach to the arene face opposite to that involved in metal coordination.
9.2.4.2 Toluene
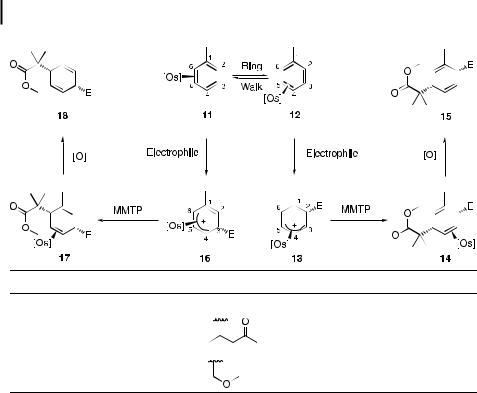
Alkylated benzenes behave similarly to benzene with respect to the stereochemistry and regiochemistry of tandem additions, but the mechanistic pathway of such additions becomes more complicated with the introduction of a methyl group. The toluene complex exists in solution as a 3:2 mixture of interconverting linkage isomers, with the C4–C5 bound arene (12) being favored over the C5–C6 bound arene (11) (Table 2). The addition of either trifluoromethanesulfonic acid (HOTf ) (entry 1) or 3-penten-2-one (entry 2) at C2 of the bound toluene generates the h3-allyl complex 13. This complex can be trapped with 1-methoxy-2- methyl-1-trimethylsilyloxypropene (MMTP), resulting in a single regioisomer, 14. Upon oxidation of 14 with AgOTf, the diene 15 is released in moderate overall yield [15]. The tandem addition of dimethoxymethane and MMTP to [Os]-toluene (entry 3) leads to a mixture of 15 and 18 after demetalation. If the tandem addition is performed at 40 C, the 15:18 ratio is 3:1. If it is performed at 70 C, the 15:18 ratio is 8:1. Although the origin of this selectivity
3029 Osmiumand Rhenium-Mediated Dearomatization Reactions with Arenes
Tab. 2. Tandem additions to toluene complexes 11 and 12.
Entry |
E |
Yield (%) |
1 |
H |
56b |
2 |
|
43b |
3 |
|
34a |
a ¼ 3:1 ratio of 15:18 at 40 C; 8:1 ratio of 15:18 at 70 C; b ¼ only 15 observed.
is unknown, Table 2 displays two possible pathways. Complex 11 can add an electrophile at C3 to generate 16, and complex 12 can add an electrophile at C2 to generate 13. It is thought that stabilization due to hyperconjugation of the methyl group in 13 favors the C2 addition pathway over the C3 addition pathway.
9.2.4.3 Xylenes
Contrary to what is observed during tandem addition reactions to [Os]-toluene (vide supra), electrophilic additions to [Os]-bound orthoand meta-xylenes result in regioselective attack at C6 (Table 3). A coordination isomer having the metal across C4–C5 (19) is the only isomer observed for both orthoand meta-xylene. Electrophilic addition of HOTf (entry 1) or dimethoxymethane (entries 2 and 3) at C6 generates the complexed allyl cation 20, which can be trapped with MMTP to form the complexed diene 21. Demetalation using AgOTf releases the free diene 22, which potentially possesses two adjacent quaternary centers (entry 3) [15].
9.2.5
Naphthalene
A one-pot tandem addition/oxidative decomplexation methodology has been developed, which yields substituted dihydronaphthalenes from [Os]-naphthalenes. The process involves
