
Astruc D. - Modern arene chemistry (2002)(en)
.pdf
14.2 Mechanistic Overview 483
Scheme 6. Phenoxonium cation generation.
The question as to whether the reactive intermediate is the phenol–metal/leaving group complex 21/22 or the free phenoxonium ion 17 has been studied in the particular case of hypervalent iodine. Pelter and co-workers presented permissive evidence in support of a mechanism involving the free oxonium species 17 (Scheme 7): PhI(OAc) is an extremely good nucleofuge, no transfer of chirality is observed when homochiral hypervalent iodine compounds are used, and calculations made on the cation species correctly predict the regioselectivity of the substitution reaction [32, 33].
Scheme 7. Phenoxonium ion generation using hypervalent iodine precursors.
Biaryl formation through oxidative coupling of phenol ethers has also been explored. Although vanadium and lead complexes are known to e ect these transformations [34], thallium reagents give the best results [35–37]. More recently, Kita and co-workers have developed the e cient oxidative coupling of phenol ethers using hypervalent iodine reagents activated by BF3 Et2O [38, 39]. The formation of a single electron transfer (SET) complex 25 is proposed to occupy a central role in the mechanism of these reactions (Scheme 8) [40]. Coupling then occurs between two cation radicals 26 or between the cation radical 26 and a neutral phenol ether molecule 24 [41].
Scheme 8. Hypervalent iodine-mediated phenyl methyl ether oxidative coupling.
It is di cult to make generalizations about the oxidative coupling of arenes, given the number of possible mechanisms. Factors that influence the reaction course include the oxidation potential of each aryl unit, the substitution pattern, the solvent system, and the oxidation reagents themselves. Optimization then becomes a case-by-case e ort in order to obtain the best selectivities and the best yields. Nevertheless, results over the last decade have


14.3 Oxidative Coupling Reactions with Hypervalent Iodine Reagents 485
Tab. 2. Substituent e ects on the PIFA-mediated intramolecular oxidative cyclization of a,o-biaryls.
R1 |
R2 |
R3 |
R4 |
R5 |
n |
X |
Yield 30 (%) |
|
|
|
|
|
|
|
|
OCH3 |
H |
OCH3 |
OCH3 |
H |
1 |
CH2 |
91 |
aOCH2Oa |
aOCH2Oa |
H |
1 |
CH2 |
91 |
||
OCH3 |
OCH3 |
OCH3 |
OCH3 |
H |
1 |
CH2 |
99 |
OCH3 |
OCH3 |
OCH3 |
OCH3 |
OCH3 |
1 |
CH2 |
92 |
OCH3 |
OCH3 |
OCH3 |
OTBS |
H |
1 |
CH2 |
75 |
OCH3 |
H |
OCH3 |
OCH3 |
H |
2 |
NCOCF3 |
89 |
OCH3 |
OCH3 |
OCH3 |
OCH3 |
H |
2 |
NCOCF3 |
68 |
OCH3 |
OCH3 |
OCH3 |
OCH3 |
OCH3 |
2 |
NCOCF3 |
52 |
aOCH2Oa |
aOCH2Oa |
H |
1 |
NCOCF3 |
94 |
||
OCH3 |
OCH3 |
OCH3 |
OCH3 |
H |
1 |
NCOCF3 |
85 |
OCH3 |
OCH3 |
OCH3 |
OCH3 |
OCH3 |
1 |
NCOCF3 |
85 |
OCH3 |
OCH3 |
OCH3 |
OTBS |
H |
1 |
NCOCF3 |
64 |
OCH3 |
OCH3 |
OCH3 |
OAc |
H |
1 |
NCOCF3 |
60 |
|
|
|
|
|
|
|
|
X ¼ CH2, 31h–k) and sulfones (Y ¼ SO2, X ¼ CH2, 31l,m) participated e ectively in the oxidative coupling reaction. Dibenzyl ethers (Y ¼ O, X ¼ CH2, 31n–r) were also coupled in fair to good yields. Cleavage of the temporary tether subsequently delivers the acyclic biaryls
33a–r.
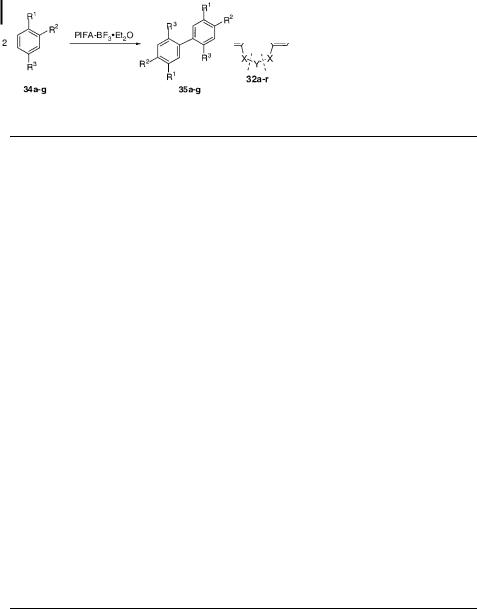
Recently, Kita and co-workers also applied the optimized PIFA/BF3 Et2O conditions to intermolecular coupling reactions [39]. Thus, several phenol ethers undergo dimerization through oxidative coupling in very good yields (Table 4). Apparently, the reaction leads to a single biaryl regioisomer. No reaction is observed when the ring is substituted by the electron-withdrawing group NO2 (substrate 34d).
For the coupling of binaphthyl compounds, the authors note that 0.55 equivalents of PIFA and a temperature below 0 C are necessary conditions to obtain the best yields (Table 5). Carbon–carbon bond formation occurs between the most highly oxygenated aryl rings of the naphthyl units.
The hypervalent iodine reagents PIFA and PIDA have also been used in the synthesis of naturally occurring structures, primarily the amaryllidaceae alkaloids and related species. Work by White’s group showed the feasibility of this method for the synthesis of 6aepipretazettine and (–)-codeine [45, 46]. In the early 1990s, Rama Krishna and co-workers demonstrated that PIDA can promote the oxidative phenolic coupling of diaryl substrates 38a–e to deliver cyclohexadienones 39a–e, respectively, in consistent 30 % yields for all of the substrates examined (Scheme 10) [47].
Kita and co-workers elaborated upon these earlier studies by examining substrates bearing a strategically placed nitrogen atom as a route to members of the amaryllidaceae alkaloid
48614 Oxidative Aryl-Coupling Reactions in Synthesis
Tab. 3. Substituent e ects in the PIFA-mediated coupling of various linked biaryls.
31 |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
X |
Y |
Yield 32 (%) |
a |
OCH3 |
OCH3 |
H |
H |
OCH3 |
OCH3 |
O |
SiiBu2 |
56 |
b |
aOCH2Oa |
H |
H |
aOCH2Oa |
O |
SiiBu2 |
69 |
||
c |
OCH3 |
OCH3 |
H |
H |
aOCH2Oa |
O |
SiiBu2 |
46 |
|
d |
OCH3 |
OCH3 |
H |
H |
OCH3 |
OCH3 |
O |
SitBu2 |
81 |
e |
aOCH2Oa |
H |
H |
aOCH2Oa |
O |
SitBu2 |
89 |
||
f |
OCH3 |
OCH3 |
H |
H |
aOCH2Oa |
O |
SitBu2 |
83 |
|
g |
OCH3 |
OCH3 |
H |
H |
OCH3 |
OCH3 |
CH2 |
S |
– |
h |
OCH3 |
OCH3 |
H |
H |
OCH3 |
OCH3 |
CH2 |
SO |
73 |
i |
aOCH2Oa |
H |
H |
aOCH2Oa |
CH2 |
SO |
71 |
||
j |
OCH3 |
OCH3 |
H |
H |
aOCH2Oa |
CH2 |
SO |
59 |
|
k |
OCH3 |
OCH3 |
OCH3 |
H |
OCH3 |
OCH3 |
CH2 |
SO |
42 |
l |
OCH3 |
OCH3 |
H |
H |
OCH3 |
OCH3 |
CH2 |
SO2 |
78 |
m |
aOCH2Oa |
H |
H |
aOCH2Oa |
CH2 |
SO2 |
72 |
||
n |
OCH3 |
OCH3 |
H |
H |
OCH3 |
OCH3 |
CH2 |
O |
85 |
o |
aOCH2Oa |
H |
H |
aOCH2Oa |
CH2 |
O |
80 |
||
p |
OCH3 |
OCH3 |
H |
H |
aOCH2Oa |
CH2 |
O |
51 |
|
q |
OCH3 |
OCH3 |
OCH3 |
H |
OCH3 |
OCH3 |
CH2 |
O |
50 |
r |
OCH3 |
OCH3 |
OCH3 |
OCH3 |
OCH3 |
OCH3 |
CH2 |
O |
38 |
Tab. 4. Intermolecular PIFA-mediated oxidative arylic coupling.
34 |
R1 |
R2 |
R3 |
Time (h) |
Yield 35 (%) |
a |
OCH3 |
OCH3 |
OCH3 |
1.5 |
92 |
b |
OCH3 |
OCH3 |
CH3 |
1.5 |
93 |
c |
OCH3 |
OCH3 |
Br |
1.5 |
97 |
d |
OCH3 |
OCH3 |
NO2 |
24 |
– |
e |
OCH3 |
CH3 |
OCH3 |
1.5 |
92 |
f |
OCH3 |
Br |
OCH3 |
1.5 |
91 |
g |
Br |
OCH3 |
H |
3 |
72 |

14.3 Oxidative Coupling Reactions with Hypervalent Iodine Reagents 487
Tab. 5. PIFA-mediated binaphthyl formation from substituted naphthyl ethers.
36 |
R1 |
R2 |
R3 |
R4 |
Time (h) |
Yield 37 (%) |
a |
H |
H |
H |
OCH3 |
3 |
91 |
b |
H |
H |
OCH3 |
OCH3 |
1.5 |
61 |
c |
Br |
H |
H |
OCH3 |
1.5 |
98 |
d |
H |
OCH3 |
H |
H |
3 |
94 |
e |
OCH3 |
H |
H |
OCH3 |
3 |
82 |
Scheme 10. Cyclohexadienone formation from PIDA-mediated oxidative coupling of phenolic substrates.
family. In 1996, they described an exploration of the coupling of norbelladine derivative 40a to a ord tricyclic compound 41a, an intermediate in the synthesis of amaryllidaceae alkaloids [22]. Apparently, the reactions only proceeded e ciently in (CF3)2CHOH at –40 C using PIFA (see Table 6) [48]. Other solvents (CH3CN, benzene, CH2Cl2, etc.) gave unsatisfactory results.
Subtle e ects on the coupling e ciency exerted by the nitrogen substituent were uncovered. No AraAr coupling products were observed with NCH3 or unprotected nitrogen (Table 7).
Further substrate variants were explored in order to ascertain the e ect of the phenoxy substituents on coupling e ciency (Table 8). The subtle influence of remote substituents is again illustrated by these results, with both more electron-rich (R1 ¼ R2 ¼ TBDMS, 40j) and more electron-deficient (R1 ¼ CH3, R2 ¼ Ac, 40n) analogues performing less satisfactorily than the parent dimethoxy ether 40a.

48814 Oxidative Aryl-Coupling Reactions in Synthesis
Tab. 6. Solvent e ects in the PIFA-mediated oxidative coupling of phenolic substrates.
Solvent |
Yield (%) |
Solvent |
Yield (%) |
|
|
|
|
(CF3)2CHOH |
70 |
Et2O |
30 |
CF3CH2OH |
61 |
DMF |
18 |
CH3CN |
50 |
THF |
15 |
C6H6 |
44 |
C6H5CH3 |
14 |
CH2Cl2 |
30 |
– |
– |
Finally, reactions with O-protected phenol 40 were studied, but only silyl ethers (40s–u, R ¼ TMS, TBDMS) a orded the cyclohexadienone product 41 in good yield. Other protecting groups primarily yielded the biaryl coupling product 42 (Table 9).
The authors proposed a mechanistic explanation for these di erent behaviors. For the case of the free phenol cyclization precursor 40a, an ionic mechanism via intermediate 44 leads to the nucleophilic intramolecular para substitution product 41a (Scheme 11). For the O- protected derivatives 40s–w, an alternative mechanism leads to the formation of the cation radical 45, which then participates in an intramolecular cyclization to deliver an activated
Tab. 7. E ect of remote nitrogen substituents on the PIFA-mediated coupling of phenolic substrates.
40 |
R |
Yield 41 (%) |
|
|
|
a |
COCF3 |
61 |
b |
CO2 tBu |
49 |
c |
CO2(CH2)2TMS |
54 |
d |
CO2Et |
48 |
e |
COC6F5 |
50 |
f |
CH3 |
– |
g |
H |
– |
|
|
|

14.3 Oxidative Coupling Reactions with Hypervalent Iodine Reagents 489
Tab. 8. E ect of various ether substituents on the PIFA-mediated coupling of phenolic substrates.
40 |
R1 |
R2 |
Yield 41 (%) |
a |
CH3 |
CH3 |
61 |
h |
|
aCH2 a |
56 |
i |
TBDMS |
CH3 |
42 |
j |
TBDMS |
TBDMS |
42 |
k |
CH3 |
TBDMS |
35 |
l |
PhCH2 |
CH3 |
49 |
m |
CH3 |
tBuCO |
32 |
n |
CH3 |
CH3CO |
37 |
o |
CH3CO |
CH3 |
Trace |
p |
H |
CH3 |
19 |
q |
CH3 |
H |
Trace |
r |
H |
H |
Trace |
|
|
|
|
Tab. 9. E ect of phenol ether substitution and solvent variation on the PIFA-mediated oxidative coupling of polyether substrates.
40 |
R |
Solvent |
Time |
41a (%) |
42 (%) |
|
|
|
|
|
|
a |
H |
CF3CH2OH |
5 min |
61 |
– |
s |
TMS |
CF3CH2OH |
30 min |
57 |
– |
t |
TBDMS |
CF3CH2OH |
4.5 h |
66 |
– |
u |
TBDPS |
CF3CH2OH |
4 h |
23 |
12 |
v |
PhCH2 |
CF3CH2OH |
24 h |
– |
48 |
w |
CH3 |
CF3CH2OH |
30 min |
– |
47 |
w |
CH3 |
(CF3)2CHOH |
1 h |
– |
42 |
w |
CH3 |
CH3CN |
3.5 h |
33 |
23 |
w |
CH3 |
CH2Cl2 |
24 h |
22 |
– |


14.3 Oxidative Coupling Reactions with Hypervalent Iodine Reagents 491
Scheme 13. Galanthamine-type alkaloids targeted by oxidative coupling methodology.
protecting groups (e.g. 50f ) was found to give the desired regioselectivity in the highest yield (Table 10). Exclusive p-p0 coupling to deliver the undesired isomer 54 was obtained when the p0 position was not blocked.
Very recently, Node and co-workers improved the e ciency of this particular oxidative phenolic coupling in the context of a synthesis of (G)-galanthamine (51a) [50]. By using a trialkoxyarene as one of the aryl units, they were able to obtain yields of 56b of up to 90 % when the nitrogen was protected with a formyl group and the donor aryl’s oxygen atoms were capped by benzyl moieties (Table 11). These authors were even able to isolate an inter-
esting narwedine-type product 57a in low yield. |
|
|
A |
similar kind of strategy, as developed by Kita, |
has also been successfully applied |
in a |
diversity-oriented synthesis of galanthamine-like |
molecules (Scheme 14) [51]. The |
Tab. 10. Regiochemical control in the PIFA-mediated oxidative coupling of unsymmetrically substituted biaryl substrates.
Substrate |
|
|
|
Isolated Yield (%) |
|
|
|
|
|
|
|
50 |
R1 |
R2 |
X |
53 (p-oO) |
54 (p-pO) |
a |
CH3 |
CH3 |
SPh |
0 |
0 |
b |
CH3 |
CH3 |
TMS |
0 |
26 |
c |
|
aCH2 a |
Br |
6 |
2 |
d |
|
aCH2 a |
SPh |
0 |
0 |
e |
|
aCH2 a |
TMS |
32 |
0 |
f |
|
aCPh2 a |
TMS |
36 |
9 |
g |
|
aC(CH3)2 a |
TMS |
46 |
12 |
h |
|
aCPh2 a |
H |
0 |
60 |
i |
|
aC(CH3)2 a |
H |
0 |
55 |
j |
|
aCPh2 a |
TES |
37 |
0 |
k |
|
aCPh2 a |
TBS |
28 |
0 |

49214 Oxidative Aryl-Coupling Reactions in Synthesis
Tab. 11. A higher-yielding PIFA-mediated oxidative cyclization approach to galanthamine-type alkaloids.
55 |
R1 |
R2 |
R3 |
Temp ( C) |
Time (min) |
Product |
Yield (%) |
a |
H |
CH3 |
COCF3 |
20 |
20 |
57a |
12 |
b |
Bn |
Bn |
CHO |
40 |
120 |
56b |
90 |
c |
CH3 |
CH3 |
CHO |
40 |
15 |
56c |
95 |
d |
Bn |
CH3 |
CHO |
40 |
60 |
56d |
82 |
d |
Bn |
CH3 |
CHO |
r.t. |
15 |
56d |
85 |
e |
Allyl |
CH3 |
CHO |
40 |
30 |
56e |
48 |
f |
MOM |
CH3 |
CHO |
40 |
10 |
56f |
43 |
g |
CH3 |
CH3 |
COCF3 |
40 |
120 |
56g |
75 |
h |
Bn |
CH3 |
COCF3 |
40 |
60 |
56h |
53 |
Scheme 14. PIFA-mediated, solid-phase synthesis of galanthamine analogue precursors.
tris(allyl)-protected precursor 58 was cyclized under standard conditions. Pelish and coworkers took good advantage of the selectivity and the mildness of the hypervalent iodine reagent to perform the coupling without a ecting the resin connection or other parts of the molecule.
In 1999, Dominguez and co-workers showed that phenanthro[9, 10-d] fused isoxazoles 61 and related pyrimidines 63 could be obtained from the biarylisoxazoles 60 and biarylpyrimidines 62, respectively, with PIFA as the oxidant [52]. This reagent proved to be the most e cient and a orded product mixtures from which the desired biaryl product could be iso-
