
574
16
Molecular Switches and Machines Using Arene Building Blocks
Hsian-Rong Tseng and J. Fraser Stoddart
Abstract
The ‘‘top-down’’ approach to device construction currently utilized by solid-state physicists and electronic engineers faces increasing challenges from the intrinsic limitations of the materials themselves. A potential solution to this rapidly emerging problem is the ‘‘bottomup’’ approach, starting from the smallest components of materials, namely molecules. A molecular-level machine is an assembly of a distinct number of molecular components that can be induced to produce mechanical movements. Similarly, a molecular switch is a molecule that can be stimulated to undergo reversible switching between discrete states. Undoubtedly, the best means of stimulating molecular-level machines and switches are through the action of photons and electrons. Thus, appropriate designs need to incorporate both electrochemically and photochemically active components into the molecules to drive the mechanical and switching processes. A working platform for these molecular-level machines and switches can be created around mechanically interlocked systems. This chapter relates a few case histories that have been inspired by the desire to construct unique molecular switches and motors, using molecules that contain certain ubiquitous arene building blocks.
16.1
Introduction
The past decade has witnessed a rapidly growing, almost obsessive, interest in molecular switches and motor-molecules that remind us of our computers and our cars. Perhaps it is little wonder that those technological triumphs, which have so changed our lifestyles on many parts of the planet during the latter half of the twentieth century have started to capture the imaginations of scientists and engineers educated and trained in how to make, measure, and model a much smaller world built up of atoms and molecules. Surely it is only natural for these scientists and engineers to ask themselves – can we make switches and motors down at the molecular level and, if we can, what could be the technological implications and consequences? These questions, which were not so long ago dismissed as somewhat academic and esoteric, have all of a sudden become rooted in the highly fashionable practice we call nanoscience and the endless speculation we embrace under the umbrella of nanotechnology. The reason for all the interest and excitement is many-fold. On the
Modern Arene Chemistry. Edited by Didier Astruc
Copyright 8 2002 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim ISBN: 3-527-30489-4

16.2 From Self-Assembling [2]Catenanes to Electronic Devices 575
one hand, molecular scientists look at the revolution that has visited the life sciences in the last two decades with the rapidly growing realization that living systems consist, in large measure, of a collection of biomolecular switches and motors that are very closely integrated, one with another in such an awesome way that life itself is not only sustained but also promulgated. On the other hand, molecular scientists are curious to see what they might find out about materials and function if they break away from pursuing the traditional disciplines within gas, solution, and solid-phase chemistry, and begin to explore the new frontiers beyond the molecule between states and across boundaries.
This chapter describes some examples of research that has been inspired by the desire to do something di erent with arene building blocks that are plentiful in their supply – and, it turns out, rich in their potential for the construction of molecular switches and motors [1].
16.2
From Self-Assembling [2]Catenanes to Electronic Devices
Our first case history starts with the establishment of a recognition motif between p-electron- rich and -deficient arene building blocks within the context of host–guest chemistry. Donor– acceptor interactions lead (Figure 1a) to the p-electron-rich macrocycle, bis(paraphenylene)- 34-crown-10 (BPP34C10) (1) accommodating the p-electron-deficient dicationic guest 22þ and forming [2] a 1:1 complex [1I2]2þ, both in solution and in the solid state. The donor– acceptor roles can be reversed [3] to give (Figure 1b) a 1:1 complex [4I3]4þ between the p- electron-rich 1,4-dimethoxybenzene (3) and the p-electron-deficient tetracationic cyclophane 44þ. During the past 15 years, these noncovalent interactions between p-electron-deficient and p-electron-rich components in recognition sites have been exploited in our laboratories to self-assemble [2]catenanes. A [2]catenane is a molecule composed of two interlocked macrocyclic components. The two macrocycles are not linked covalently to each other; rather, a mechanical bond holds the two components together, preventing their dissociation.
Fig. 1. Two complementary p-electron-donor/acceptor recognition systems, [1I2]2þ and [4I3]4þ.
576 16 Molecular Switches and Machines Using Arene Building Blocks
Fig. 2. The template-directed synthesis of the [2]catenane 5 4PF6.
As shown in Figure 2, the template-directed synthesis of a [2]catenane 5 4PF6, which relies on donor–acceptor interactions for the initial self-assembly of its component pieces prior to the formation of the mechanical bond, proceeds [4] in 70 % yield when equimolar amounts of 6 2PF6 and 1,4-bis(bromomethyl)benzene (7) are reacted together in the presence of an excess of BPP34C10 (1). This [2]catenane is composed of a p-electron-deficient tetracationic cyclophane interlocked with a p-electron-rich macrocyclic polyether. In addition to its having
amechanical bond, [p p] stacking interactions [5] between the complementary aromatic units, [CaH O] hydrogen bonds [6] between a-bipyridinium hydrogen atoms and polyether oxygen atoms, and [CaH p] interactions [7] between the hydrogen atoms on the hydroquinone (HQ) ring and the p-phenylene spacer in the tetracationic cyclophane hold the two macrocyclic components together and also control their relative movements in solution.
On the basis of the same kinds of p-electron donor–acceptor interactions, and in collaboration with the Balzani group in Bologna, a more complicated (supramolecular) system has been built [8] from three components. Two components behave as hosts with di ering p- donor/p-acceptor capabilities, and the other component fulfils the role of an itinerant guest with redox-controllable p-donor/p-acceptor properties. Three distinct states can be accessed (Figure 3) by electrochemical manipulation of the guest’s p-donor/p-acceptor properties in the presence of the two hosts, namely the p-electron-donating macrocyclic polyether, 1,5- dinaphtho-38-crown-10 (DN38C10) (8) and the p-electron-accepting tetracationic cyclophane 44þ. The guest is tetrathiafulvalene (TTF) (9), which is known to exist in three stable oxidation states, namely TTF(0), TTFþ , and TTF2þ. The three-component mixture functions as
athree-pole system by controlling the oxidation state of the TTF unit. The TTF is (i) uncomplexed in its TTFþ state, (ii) complexed with the p-electron-accepting tetracationic cyclophane 44þ in its TTF(0) state, and (iii) complexed with the p-electron-donating macrocyclic polyether 8 in its TTF2þ state. The reversible electrochemical processes were monitored by absorption spectroscopy. The results show that complexation/decomplexation occurs rapidly on the experimental time-scale.
The [2]catenane 104þ, incorporating a TTF unit in its p-electron-rich macrocyclic ring, has been self-assembled [9a, 9b] using the template-directed synthetic strategy (Figure 4). The bis(hexafluorophosphate) salt 6 2PF6 was treated with 1,4-bis(bromomethyl)benzene (7) in the presence of the macrocycle 11 to obtain 10 4PF6, which was isolated in a yield of 23 % after counterion exchange. X-ray crystallography revealed (Figure 5) that the TTF unit resides preferentially inside the cavity of the tetracationic cyclophane in the solid state. This observation is consistent with the absorption spectrum of 10 4PF6 in MeCN. It shows a band at
16.2 From Self-Assembling [2]Catenanes to Electronic Devices 577
Fig. 3. A three-pole supramolecular switch.
850 nm (curve a in Figure 6), corresponding to a charge-transfer (CT) interaction between the TTF unit and the bipyridinium units. The circumrotation of the macrocyclic polyether component through the cavity of the tetracationic cyclophane can be reversibly induced by oxidizing and then reducing the TTF unit. Upon addition of one equivalent of Fe(ClO4)3, the TTF unit is oxidized (from A to B in Figure 6) to its radical cation. Circumrotation of the macrocycle occurs as a result of electrostatic repulsion between this monocationic unit and the two dicationic bipyridinium units. This circumrotation can be monitored by observing the changes in the absorption spectrum. The CT band, centered at 850 nm, gradually dis-
Fig. 4. The template-directed synthesis of the [2]catenane 10 4PF6.

578 16 Molecular Switches and Machines Using Arene Building Blocks
Fig. 5. The X-ray crystal structure of the [2]catenane 104þ.
appears and is replaced by a new CT band, centered at 515 nm (curves b and c in Figure 6), arising from the 1,5-dihydroxynaphthalene (DNP) ring system and the surrounding bipyridinium units. Again, upon addition of a second equivalent of Fe(ClO4)3, the monocationic (TTFþ ) unit is oxidized (from B to C in Figure 6) to its dication (TTF2þ). The absorption spectrum shows a similar CT band centered at 515 nm (curve d in Figure 6), suggesting that the DNP ring system still resides inside the cavity of the tetracationic cyclophane. Upon addition of two equivalents of ascorbic acid, the TTF2þ dication is reduced back to its neutral state (from C to A in Figure 6) and the macrocyclic polyether once again circumrotates through the cavity of the tetracationic cyclophane, returning to the original state of the [2]catenane. Absorption spectroscopy corroborates this sequence of events by showing the same band (curve e in Figure 6) at 850 nm as that present in the starting [2]catenane 10 4PF6. The circumrotation of the macrocyclic polyether component through the cavity of the tetracationic cyclophane can also be induced electrochemically. In cyclic voltammetry
Fig. 6. The absorption spectra of the redox processes of the [2]catenane 10 4PF6.

16.2 From Self-Assembling [2]Catenanes to Electronic Devices 579
(CV) experiments [9a, 9b], the bielectronic oxidation process occurs on the TTF unit at þ0.80 V vs. saturated calomel electrode (SCE), and the oxidation of the DNP ring system is strongly displaced toward þ1.60 V vs. SCE, compared to that of the free crown ether (þ1.17 V vs. SCE). The dramatic increase in the oxidation potential of the DNP ring system indicates that it is encircled by the tetracationic cyclophane after the oxidation of the TTF unit.
The dimiristoylphosphatidyl (DMPA) salt of the switchable [2]catenane 104þ forms [9c] a stable Langmuir–Blodgett (LB) monolayer at the air–water interface. The monolayers can be transferred onto an Au(111) surface and have been studied [9d] by scanning tunneling spectroscopy. The current/voltage behavior of the resulting materials is dictated by the redox state of the TTF unit and by the co-conformation of the [2]catenane. No current is detected between 0.5 V and 0.0 V when the TTF unit is initially in its neutral state and located inside the cavity of the tetracationic cyclophane. However, relatively high tunneling currents are obtained when the TTF unit is initially in the oxidized state and located alongside the cavity of the tetracationic cyclophane.
In collaboration with the Heath group at UCLA, the [2]catenane 10 4DMPA has been introduced [9e] into electronically reconfigurable molecular switches. By employing the LB technique, monolayers of the DMPA salt of the switchable [2]catenane were transferred onto a parallel arrangement of aligned n-type polysilicon wires, supported by a SiO2 substrate. The 7 mm wide wires were deposited onto the substrate by direct chemical vapor deposition. A second set of wires, perpendicular to the first, was then deposited onto the monolayer through a contact shadow mask, using an electron beam deposition technique. These wires were composed of a layer of Ti (5 nm) and Al (100 nm) and had a width of 10 mm. A typical device is illustrated graphically in Figure 7. In this illustration, an Al/Ti wire overlays six polysilicon wires, forming a linear array of six junctions. A section of these junctions is magnified in Figure 7, which shows the two perpendicular wires sandwiching a film of the DMPA anion lying on top of a monolayer of switchable [2]catenane molecules. By alternating the voltage pulses between þ2 V and 2 V, and reading the resistance at 0.1 V, switching between the two states of the [2]catenane is observed. The remnant molecular signature of the device, which is shown in Figure 8, was measured by varying the writing voltage in 40 mV steps and reading the device at 0.2 V. The hysteresis between the open and closed states of the catenane-based switch is evident in this signature. This electronically recon-
Fig. 7. The electronically reconfigurable molecular devices based on the [2]catenane 10 4DMPA.
580 16 Molecular Switches and Machines Using Arene Building Blocks
Fig. 8. The remnant molecular signature of the [2]catenane device, measured by varying the write voltage in 40 mV steps and by reading the device at 0.2 V.
figurable bistable catenane-based switch might be useful for generating random access memory.
16.3
A Hybrid [2]Catenane Switch
In collaboration with the Sauvage group in Strasbourg, we have been successful in combining [10] metal–ligand and p-electron donor–acceptor interactions to produce (Figure 9) a [2]catenane [12 Cu]5þ that is capable of chemical switching. The threaded complex [14I13 Cu]3þ was formed by mixing equimolar amounts of Cu(MeCN)4PF6, the dication 132þ, and the phenanthroline-containing macrocycle 14. Treating the threaded complex
Fig. 9. The synthesis and chemical switching of a hybrid [2]catenane.
16.4 A Self-Complexing Molecular Switch 581
[14I13 Cu]3þ with 1,3-bis(bromomethyl)benzene (15) under high dilution conditions in refluxing MeCN, gave the [2]catenane [12 Cu]5þ in 40 % yield. This hybrid molecular switch possesses two di erent recognition modes, namely (i) coordination of Cu(I) by two phenanthroline ligands and (ii) p-electron donor–acceptor interactions between the DNP ring system and the two bipyridinium units. Since the coordination interaction is the stronger, the stable state of the molecular switch is the [2]catenane [12 Cu]5þ, in which the Cu(I) ion is complexed by the two phenanthroline ligands in a tetrahedral coordination geometry. Removal of the Cu(I) ion, however, by the addition of KCN, a ords the [2]catenane 124þ, which undergoes a topological change to allow its p-electron-rich DNP ring system to be sandwiched between its two p-electron-deficient bipyridinium units. Thus, non-covalent donor– acceptor interactions dominate the molecular geometry in the demetalated system. The chemically driven co-conformational change between the [2]catenanes [12 Cu]5þ and 124þ was confirmed by 1H NMR spectroscopy.
16.4
A Self-Complexing Molecular Switch
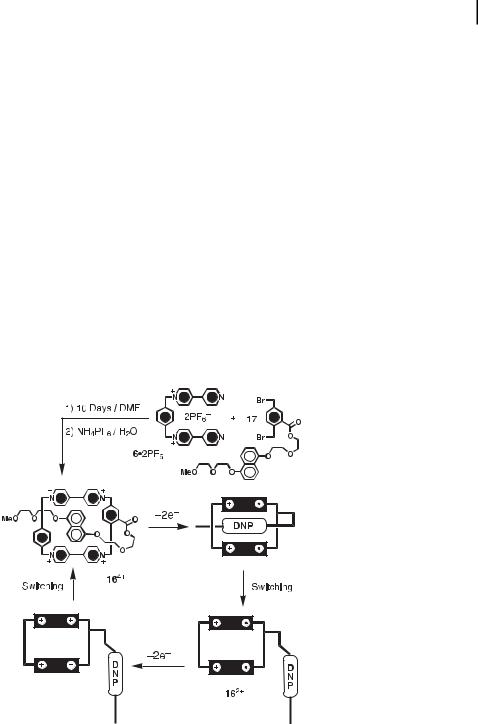
Our next case history takes what we have learnt about donor–acceptor interactions between arene building blocks in interlocked molecules and exploits that knowledge base in a more conventional intramolecular arena. The self-complexing compound 164þ (Figure 10) incorporates [11] a linear polyether thread intercepted by a DNP ring system, which is co-
Fig. 10. The synthesis and switching of the self-complexing compound 164þ.
58216 Molecular Switches and Machines Using Arene Building Blocks
valently bound to one of the phenylene spacers of the tetracationic cyclophane by means of an ester linkage. In solution, the DNP ring system threads through the cavity of the cyclophane. The conformation is stabilized by p-p stacking interactions between the complementary p-electron-rich DNP ring system and the two p-electron-deficient bipyridinium units in
the tetracationic cyclophane. By reacting the dication 6 2PF6 with the DNP-containing dibromide 17, the self-complexing macrocycle 164þ was obtained in 24 % yield. The absorption spectrum of the macrocycle 164þ, recorded in MeCN at 298 K, shows a band centered at 515
nm, corresponding to the CT interaction between a DNP ring system and the sandwiching bipyridinium units. The absorption increases linearly with the concentration, suggesting that this compound exists only in the self-complexing form. The compound also functions as an electrochemically driven molecular switch. After electrochemical reduction of each bipyridinium unit of the cyclophane, the DNP-containing side chain is ejected from the cyclophane’s cavity. This dramatic conformational change is reversible by means of the electrochemical redox process. The switching process was monitored by CV and indeed proved to be reversible.
16.5
Pseudorotaxane-Based Supramolecular Machines
The recognition of di erent p-donating arene guests with a common p-accepting host has been subjected to control by metal ion crown ether recognition in the next case history to be described. The 18-crown-6 derivative 18, which bears a DNP ring system [12], is a ditopic compound that can act (Figure 11) as a host for alkali metal (e.g., Kþ) cations as well as as a
Fig. 11. The chemically controlled competition between two thread-like species 18 and 19 for the cavity of a tetracationic cyclophane 44þ.










