
The Elisa guidebook
.pdf
Page viii
ing proteins. An excellent manual covering all aspects of immunochemistry is available [Harlow and Lane (23)], which also outlines many relevant laboratory practices.
JOHN R. CROWTHER, PHD
References
1.Avrameas, S. (1966) Methode de marquage d'antigenes et d'anticorps avec des enzymes et son application en immunodiffusion. Comptes Rendus Hendomadaires des Seances de l'Acadamie des Sciences: D: Sciences naturelles (Paris), 262, 2543¨C2545.
2.Nakane, P. K. and Pierce, G. B. (1966) Enzyme-labelled antibodies: preparation and application for the localization of antigens. J. Histochem. Cytochem. 14, 929¨C931.
3.Avrameas, S. (1969) Coupling of enzymes to proteins with gluteraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry 6, 43¨C52.
4.Avrameas, S. and Guilbert, B. (1971) Dosage enzymo immunologique de proteines a l'aide d'immunosadorbants et d'antigenes marques aux enzymes. Comptes Rendus Hendomadaires des Seances de l'Acadamie des Sciences: D: Sciences naturelles (Paris), 273, 2705¨C2707.
5.Engvall, E. and Perlman, P. (1971) Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 8, 871¨C874.
6.Van Weeman, B. K. and Schuurs, A. H. W. M. (1971) Immunoassay using antigen-enzyme conjugates. FEBS Lett. 15, 232¨C236.
7.Voller, A., Bidwell, D. E., Huldt, G., and Engvall, E. (1974) A microplate method of enzyme linked immunosorbent assay and its application to malaria. Bull. World Health Organ. 51, 209.
8.Burgess, G. W. (ed.) (1988) ELISA technology in diagnosis and research. Graduate School of Tropical Veterinary Science, James Cook University of North Queensland, Townsville, Australia.
9.Collins, W. P. (1985) Alternative Immunoassays. Wiley, Chichester, UK.
10.Collins, W. P. (1985) Complimentary Immunoassays. Wiley, Chichester, UK.
11.Crowther, J. R. (1995) ELISA Theory and Practice. Humana Press, Totowa, NJ.
12.Ishikawa, E., Kawia, T., and Miyai, K. (1981)Enzyme Immunoassay. Igaku-Shoin, Tokyo, Japan.
13.Kemeny, D. M. and Challacombe, S. J. (1988) ELISA and Other Solid-Phase Immunoassays. Theoretical and Practical Aspects. Wiley, Chichester, UK.
14.Maggio, T. (1979) The Enzyme Immunoassay. CRC, New York.
15.Ngo, T. T. and Leshoff, H. M. (1985) Enzyme-Mediated Immunoassay. Plenum, New York.

16.Voller, A., Bidwell, D. E., and Bartlett, A. (1979) The Enzyme-Linked Immunosorbent Assay (ELISA). Dynatech Europe, UK.
17.Avrameas, S., Ternynck, T., and Guesdon, J. L. (1978) Coupling of enzymes to antibodies and antigens. Scand. J. Immunol. 8(Suppl 7), 7¨C23.
18.Blake, C. and Gould, B. J. (1984) Use of enzymes in immunoassay techniques. A review. Analyst
109, 533.
Page ix
19.Guilbault, G. G. (1968) Use of enzymes in analytical chemistry. Anal. Chem. 40, 459.
20.Kemeny, D. M. and Challacombe, S. J. (1986) Advances in ELISA and other solid-phase immunoassays. Immunol. Today 7, 67.
21.Voller, A., Bartlett, A., and Bidwell, D. E. (1981) Immunoassays for the 80s. MTP Press, Lancaster, UK, pp. 457¨C479.
22.Roitt, I. M., Brostoff, J., and Male, D. K. (1993) Immunology, 3rd ed. Mosby, St. Louis, MO.
23.Harlow, E. and Lane, D. (eds.) (1988) Antibodies. A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Page 1
1¡ª
Overview of ELISA in Relation to Other Disciplines
This chapter examines what areas of science are needed to allow optimal use of ELISA and notes their relationships. This information is useful for students and those instructing students. Diagrams, with brief descriptions of key points, are used to illustrate such relationships. Inherent in this exercise are considerations of the exact requirements by the operators in using the ELISA. Attention to increasing knowledge in those areas highlighted is essential both in developmental work to produce a working ELISA and in the ultimate value of any test devised. A good deal of attention should be directed at defining, as clearly as possible, the objectives for the ELISA. The development of a diagnostic test for a specific disease requires that all other data pertaining to the biology of that disease, e.g., antigenicity and structure of the agent, antibody production in different animals following infection, qualitative assessment of antibodies by different assays, and availability of standard or control sera, are known. Some attention must be paid to the laboratory facilities available, e.g., equipment, reagents already developed, small laboratory animals, experimental large animals, cash to buy commercial products, and trained personnel. In this way, the chances of producing a sustainable test to solve the defined problem are significantly greater than when a test is developed by a dabbling technique with poor or no forward planning.
Figure 1 emphasizes that we are considering the heterogeneous ELISA involving separation steps and a solid phase. Four major advantages of ELISA are promoted, all of which add to the reasons that this form of ELISA has been, and will continue to be, successful.
Figure 2 deals with the systematics of the ELISA and shows the various stages needed and factors important in those stages.
Figure 3 emphasizes that using the equipment to perform ELISAs requires skills, and that both physical and mental processes are needed. Figure 3 also indicates that instruments need to be maintained for optimal performance.
Page 2
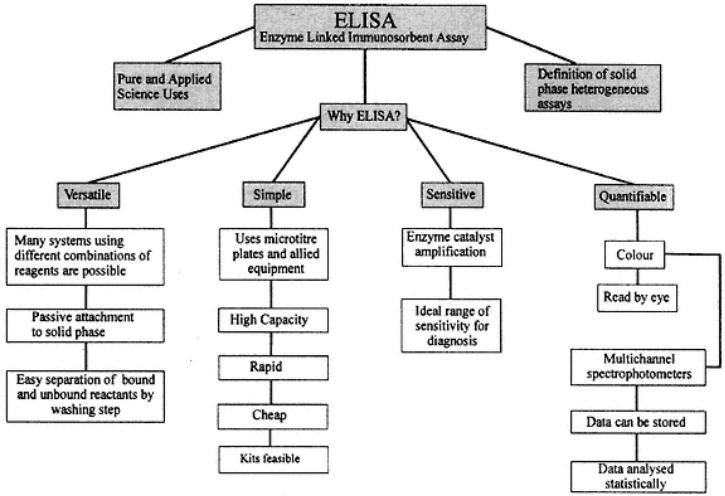
Fig. 1.
Scheme showing features of ELISA that make it advantageous for a wide range of applications.
Figure 4 deals with some of the enzymatic systems in the ELISA, and illustrates areas that need to be understood in order to allow optimal performance to be maintained. Understanding enzyme kinetics, catalysis reactions, hazards, and buffer formulation (pH control) are all essential.
Figure 5 illustrates the use of ELISAs in binding and inhibition/competition interactions to allow an understanding of a problem. It is essential that the chemical and physical nature of antibodies and antigens are understood, particularly in cases of developmental work. As full an understanding of the antigenic properties of agents being examined is needed to allow maximum exploitation of ELISA, particularly if the results are ever to be understood.
Figure 6 deals with data processing and analysis. Various essential statistical parameters must be elucidated, if data are to be interpreted. This is true in understanding how to calculate the variance in a result, and also for examining populations. Such studies actually define any ELISA's performance, allowing confidence in results to be measured, thereby allowing a meaning to be placed on results. The concepts of controlling assays with references to standards is also needed.
Figure 7 extends the use of statistical understanding into epidemiological needs. A common use of ELISA is to provide data on populations studied. The

Page 3
Fig. 2.
Scheme relating stages in ELISA. Specific stages vary according to the system utilized.

areas of sampling (size, number, and so forth) are vital when planning disease control strategies.
These simplified overviews should be used as reference points when considering the development and specific use of any ELISA. They should help
Page 4
Fig. 3.
Scheme relating equipment needs and skills for ELISA.

Fig. 4.
Relationships of enzyme systems to components of ELISA.
readers with limited exposure to ELISA, particularly after studying the details in later chapters. They are also useful for trainers in establishing areas of competence in students.
Page 5

Fig. 5.
Requires features in immunological understanding in order to establish ELISA.
These are the key points to keep in mind at this early stage when considering then use of ELISA:
1.The ELISA is a tool to solve a problem.
2.Any problem should be defined, as clearly as possible, with reference to all previous work defining the specific agent involved and related agents.
3.Other methods for analyzing the problem should be reviewed, particularly when tests are already established. This has implications if the ELISA is to replace existing tests.

4. The capacity for testing has to be addressed. For example, when an ELISA may be used on a large scale (kit), then sufficient reagents, standard sera, conjugates (batches), and antigen preparations must be available. Research leading to suc-
Page 6
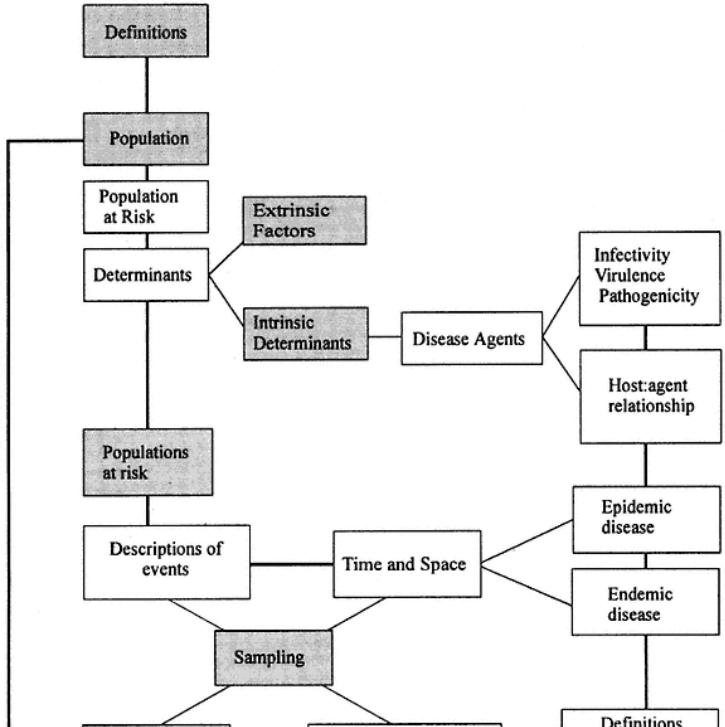
Fig. 6.
Important statistical factors needed to make use of ELISA. Note the links to quality control (internal) and the establishment of confidence in test results. Increasingly, assays need international recognition.
cessful assays in which reagents are difficult to prepare on a large scale, require extensive expertise to formulate, or are reliant on a specific limited batch of a commercial reagent are not sustainable.
5. When a test may be of use to a wider group of scientists, the possible conditions (laboratory facilities, expertise) should be considered when developing assays. Such technology transfer factors are relevant, particularly in laboratories in developing countries.
Page 7
