
- •Table of Contents
- •Preface
- •Contributors
- •1. INTRODUCTION
- •2. HIERARCHIES OF AB INITIO THEORY
- •2.3. Computational Cost
- •3.2. The CCSD(T) Model
- •4.1. Electronic and Nuclear Contributions
- •4.2. Dependence on the AO Basis Set
- •5.2. Extrapolations from Principal Expansions
- •6. CALIBRATION OF THE EXTRAPOLATION TECHNIQUE
- •6.2. Total Electronic Energy
- •6.3. Core Contributions to AEs
- •7. MOLECULAR VIBRATIONAL CORRECTIONS
- •8. RELATIVISTIC CONTRIBUTIONS
- •9. CALCULATION OF ATOMIZATION ENERGIES
- •10. CONCLUSIONS AND PERSPECTIVES
- •2. STEPS IN THE W1 AND W2 THEORIES, AND THEIR JUSTIFICATION
- •2.1. Reference Geometry
- •2.2. The SCF Component of TAE
- •2.3. The CCSD Valence Correlation Component of TAE
- •2.4. Connected Triple Excitations: the (T) Valence Correlation Component of TAE
- •2.6. Scalar Relativistic Correction
- •3. PERFORMANCE OF W1 AND W2 THEORIES
- •3.2. Electron Affinities (the G2/97 Set)
- •3.4. Heats of Formation (the G2/97 Set)
- •3.5. Proton Affinities
- •4. VARIANTS AND SIMPLIFICATIONS
- •4.2. W1h and W2h Theories
- •4.5. W1c Theory
- •4.6. Detecting Problems
- •5. EXAMPLE APPLICATIONS
- •5.1. Heats of Vaporization of Boron and Silicon
- •5.2. Validating DFT Methods for Transition States: the Walden Inversion
- •5.3. Benzene as a ”Stress Test” of the Method
- •6. CONCLUSIONS AND PROSPECTS
- •1. INTRODUCTION
- •2. THE G3/99 TEST SET
- •4. G3S THEORY
- •5. G3X THEORY
- •6. DENSITY FUNCTIONAL THEORY
- •7. CONCLUDING REMARKS
- •1. INTRODUCTION
- •2. PAIR NATURAL ORBITAL EXTRAPOLATIONS
- •3. CURRENT CBS MODELS
- •4. TRANSITION STATES
- •5. EXPLICIT FUNCTIONS OF THE INTERELECTRON DISTANCE
- •7. NEW DEVELOPMENTS
- •7.1. The SCF Limit
- •7.2. The CBS Limit for the MP2 Correlation Energy
- •7.4. Total Energies
- •8. ENZYME KINETICS AND MECHANISM
- •9. SUMMARY
- •1. INTRODUCTION
- •2. ELECTRON PROPAGATOR CONCEPTS
- •3. AN ECONOMICAL APPROXIMATION: P3
- •4. OTHER DIAGONAL APPROXIMATIONS
- •5. NONDIAGONAL APPROXIMATIONS
- •7. P3 TEST RESULTS
- •7.1. Atomic Ionization Energies
- •7.2. Molecular Species
- •8. CONCLUSIONS AND PROSPECTUS
- •1. INTRODUCTION
- •2. THEORETICAL PROCEDURES
- •3. GEOMETRIES
- •4. HEATS OF FORMATION
- •5. BOND DISSOCIATION ENERGIES
- •6. RADICAL STABILIZATION ENERGIES
- •7. REACTION BARRIERS
- •8. REACTION ENTHALPIES
- •9. CONCLUDING REMARKS
- •1. INTRODUCTION
- •2. HOMOLEPTIC CARBONYL COMPLEXES
- •4. IRON CARBONYL COMPLEXES
- •5. GROUP-10 CARBONYL COMPLEXES
- •7. NOBLE GAS COMPLEXES
- •8. TRANSITION METAL CARBENE AND CARBYNE COMPLEXES
- •12. TRANSITION METAL METHYL AND PHENYL COMPOUNDS
- •13. TRANSITION METAL NITRIDO AND PHOSPHIDO COMPLEXES
- •15. MAIN GROUP COMPLEXES OF BeO
- •16. CONCLUSION
- •1. INTRODUCTION
- •2. THEORETICAL BACKGROUND
- •3. SPECIFIC CONVENTIONS
- •4. STATISTICAL EVALUATIONS
- •5. DISCUSSION
- •Index
P3 Electron Propagator Approximations |
155 |
8.CONCLUSIONS AND PROSPECTUS
P3 calculations with unrestricted Hartree-Fock reference states have been reported here for the first time. In addition, a P3 procedure for electron affinities of closed-shell and open-shell systems has been presented.
Some general trends may be discerned in the P3 results on atoms. Ionization energies involving high-spin states are described well with Pople, Dunning, ANO, and ECP basis sets. Average errors for the p block elements are between 0.25 eV for Dunning’s  basis and 0.82 eV for the LANL
basis and 0.82 eV for the LANL 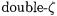 set. For the alkali and alkaline earth metals, the average errors are smaller. Transition metals in the fourth period require use of ANO or SDD sets; the average errors are 0.7 - 0.8 eV. Despite the complex character of electron correlation in 2p and 3d elements, reasonable results obtain for this simple electron propagator approximation based on an unrestricted Hartree-Fock reference state. For transition metal complexes with high oxidation states and highly electronegative ligands, one may expect the errors for metal-centered holes to be smaller. Results of test calculations using SDD ECPs are especially encouraging for this class of molecules, especially if a closedshell reference state may be used. Larger errors may be expected for late transition metals, low oxidation states, and relatively electropositive ligands. The P3 method may be used to aid state assignments in photoelectron spectra of organometallics.
set. For the alkali and alkaline earth metals, the average errors are smaller. Transition metals in the fourth period require use of ANO or SDD sets; the average errors are 0.7 - 0.8 eV. Despite the complex character of electron correlation in 2p and 3d elements, reasonable results obtain for this simple electron propagator approximation based on an unrestricted Hartree-Fock reference state. For transition metal complexes with high oxidation states and highly electronegative ligands, one may expect the errors for metal-centered holes to be smaller. Results of test calculations using SDD ECPs are especially encouraging for this class of molecules, especially if a closedshell reference state may be used. Larger errors may be expected for late transition metals, low oxidation states, and relatively electropositive ligands. The P3 method may be used to aid state assignments in photoelectron spectra of organometallics.
Results on molecules and molecular ions display some instructive tendencies. Electron detachment energies from closed-shell reference states (neutral or anionic) are treated well if there is little multiconfigurational character in the initial singlet. Average errors for these cases are about 0.2 - 0.4 eV. The quality of electron attachment energies to closed-shell species is better, especially if the singlet is cationic. Another new class of P3 calculations makes use of unrestricted Hartree-Fock reference states. If the reference state is a doublet, electron detachment energies are treated well, with average errors of 0.2 - 0.3 eV. Electron attachment energies to doublets are satisfactory if the reference state is cationic; caution must be exercised if the doublet reference state is neutral.
Although P3 procedures perform well for a variety of atomic and molecular species, caution is necessary when applying this method to open-shell reference states. Systems with broken symmetry in unrestricted Hartree-Fock orbitals should be avoided. Systems with high multireference character are unlikely to be described well by the P3 or any other diagonal approximation. In such cases, a renormalized elec-
156 Chapter 5
tron propagator should be used. In general, P3 will fail when HartreeFock theory does not provide a qualitatively acceptable description of the reference state.
The pole strength is a useful diagnostic criterion of problematic cases. Close agreement with experiment in the presence of a pole strength that is less than 0.80 is likely to be the result of a fortuitous cancellation of errors.
The P3 methods for ionization energies and electron affinities provide useful, correlated corrections to canonical Hartree-Fock orbital energies. Their computational demands are modest, especially for electron detachment energies. It is often possible to avoid difficult cases by reversing the labels of initial and final states. Fifth-power arithmetic scaling factors characterize the bottleneck contractions in P3 calculations. Full integral transformations to the Hartree-Fock basis and storage of the largest blocks of integrals may be avoided. In general, P3 calculations may be executed for any molecule where a second-order total energy calculation is feasible. Information on excited final states may be obtained easily. Interpretation of the results in terms of orbitals is facilitated by the diagonal self-energy approximation, where each Dyson orbital is equal to a canonical Hartree-Fock orbital times a scaling factor which is equal to the square root of the corresponding pole strength.
ACKNOWLEDGEMENTS
We thank Dr. Yasuteru Shigeta for informative conversations and assistance in the preparation of this manuscript. This work was supported by the National Science Foundation under grant CHE-9873897 and by the Kansas DEPSCoR program.
REFERENCES
l.J. Linderberg and Y. Öhrn, Propagators in Quantum Chemistry, Academic Press, New York (1973).
2.B. T. Pickup and O. Goscinski, Mol. Phys. 26 1013 (1973).
3.L. S. Cederbaum and W. Domcke, Adv. Chem. Phys. 36, 206 (1977).
4.J. Simons, Annu. Rev. Phys. Chem. 28, 1 (1977).
5.J. Simons, Theor. Chem. Adv. Persp. 3, 1 (1979).
6.M. F. Herman, K. F. Freed, and D. L. Yeager, Adv. Chem. Phys. 48, 1 (1981).
7.Y. Öhrn and G. Born, Adv. Quant. Chem. 13, 1 (1981).
P3 Electron Propagator Approximations |
157 |
8.W. von Niessen, J. Schirmer and L. S. Cederbaum, Comput. Phys. Rep. 1, 57 (1984).
9.J. V. Ortiz in Computational Chemistry: Reviews of Current Trends, Vol. 2, J. Leszczynski (Ed.), World Scientific, Singapore (1997) p. 1.
10.J. V. Ortiz in Conceptual Perspectives in Quantum Chemistry, Vol. 3, J.-L. Calais and E. Kryachko (Eds.), Kluwer, Dordrecht (1997) p. 465.
11.J. V. Ortiz Adv. Quantum Chem. 35, 33 (1999).
12.J. V. Ortiz, J. Chem. Phys. 104, 7599 (1996).
13.J. V. Ortiz and V. G. Zakrzewski, J. Chem. Phys. 105, 2762 (1996).
14.V. G. Zakrzewski, and J. V. Ortiz, J. Phys. Chem. 100, 13979 (1996).
15.V. G. Zakrzewski, O. Dolgounitcheva, and J. V. Ortiz, J. Chem. Phys. 105, 8748 (1996).
16.V. G. Zakrzewski and J. V. Ortiz, J. Mol. Struct. (Theochem) 388, 351 (1996).
17.J. V. Ortiz, Int. J. Quantum Chem. 63, 291 (1997).
18.M. Enlow, J.V. Ortiz and H.P. Lüthi, Molecular Physics 92, 441 (1997).
19.O. Dolgounitcheva, V. G. Zakrzewski, and J. V. Ortiz, Int. J. Quantum Chem. 65, 463 (1997).
20.O. Dolgounitcheva, V. G. Zakrzewski, and J. V. Ortiz, J. Phys. Chem. A 101, 8554 (1997).
21.V. G. Zakrzewski, O. Dolgounitcheva, and J. V. Ortiz, J. Chem. Phys. 107, 7906 (1997).
22.O. Dolgounitcheva, V. G. Zakrzewski, and J. V. Ortiz, J. Chem. Phys. 109, 87 (1998).
23.O. Dolgounitcheva, V. G. Zakrzewski, and J. V. Ortiz, Int. J. Quantum Chem. 70, 1037 (1998).
24.V. G. Zakrzewski, O. Dolgounitcheva, and J. V. Ortiz, Int. J. Quantum Chem. 75, 607 (1999).
25.O. Dolgounitcheva, V. G. Zakrzewski, and J. V. Ortiz, Int. J. Quantum Chem. 80, 831 (2000).
26.O. Dolgounitcheva, V. G. Zakrzewski, and J. V. Ortiz, J. Phys. Chem. A 104, 10032 (2000).
27.O. Dolgounitcheva, V. G. Zakrzewski, and J. V. Ortiz, J. Amer. Chem. Soc. 122, 12304 (2000).
28.J. M. Herbert and J. V. Ortiz, J. Phys. Chem. A104, 11786 (2000).
29.H. Hopper, M. Lococo, O. Dolgounitcheva, V. G. Zakrzewski, and J. V. Ortiz,
J. Amer. Chem. Soc. 122, 12813 (2000).
30.V. G. Zakrzewski, O. Dolgounitcheva, and J. V. Ortiz, Int. J. Quant. Chem. 75, 607 (1999).
31.V. G. Zakrzewski, J. V. Ortiz, J. A. Nichols, D. Heryadi, D. L. Yeager, and J. T. Golab, Int. J. Quant. Chem. 60, 29 (1996).
32.J. Simons and W. D. Smith, J. Chem. Phys. 58, 4899 (1973).
33.G. D. Purvis and Y. Öhrn, Chem. Phys. Lett. 33, 396 (1975).
158 |
Chapter 5 |
34.L. S. Cederbaum, W. Domcke, J. Schirmer, and W. von Niessen, Adv. Chem. Phys. 65, 115 (1986).
35.J. V. Ortiz, J. Chem. Phys. 108, 1008 (1998).
36.O. Dolgounitcheva, V. G. Zakrzewski, and J. V. Ortiz, J. Chem. Phys. 114, 130 (2001).
37.J. T. Golab and D. L. Yeager, J. Chem. Phys. 87, 2925 (1987).
38.D. Heryadi and D. L. Yeager, J. Chem. Phys. 114, 5124 (2001).
39.J. V. Ortiz, J. Chem. Phys. 109, 5741 (1998).
40.J. V. Ortiz, Int. J. Quant. Chem. 70, 651 (1998).
41.J. V. Ortiz, Chem. Phys. Lett. 296, 494 (1998).
42.J. V. Ortiz, Int. J. Quant. Chem. 75, 615 (1999).
43.J. V. Ortiz, Chem. Phys. Lett. 297, 193 (1998).
44.J. Lin, C. Yu, S. Peng, I. Akiyama, K. Li, L. K. Lee, and P. R. Lebreton, J. Phys. Chem. 84, 1006 (1980).
45.GAUSSIAN 98 (Revision A8), M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, Jr., R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Me nnucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko,
P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Head-Gordon, E.
S.Replogle, and J. A. Pople, Gaussian, Inc., Pittsburgh PA, 1998.
46.Contact J. V. Ortiz at ortiz@ksu.edu for specific information.
47.L. A. Curtiss, P. C. Redfern, K. Raghavachari, and J. A. Pople, J. Chem. Phys. 109, 42 (1998).
48.GAUSSIAN 99, DEVELOPMENT VERSION (REVISION B.06+), M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, Jr., R. E. Stratmann, J. C. Burant, S. Dapprich,
J.M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, C. Adamo, J. Jarmillo, R. Cammi, C. Pomelli,
J. Ochterski, G. A. Petersson, P. Y. Ayala, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S. Replogle, and J. A. Pople, Gaussian, Inc., Pittsburgh PA, 1998.
49.Basis sets (excluding the ANO category) are specified according to the Gaussian 98 keyword used to invoke their use. See A. Frisch and M. J. Frisch, Gaussian 98 User’s Reference (Gaussian, Inc., Pittsburgh, 1998) pp. 25-28 and references therein for a complete description of these basis sets.
50.Additional basis sets were obtained from the Extensible Computational Chemistry Environment Basis Set Database, June 26, 2000 Version, as developed and
P3 Electron Propagator Approximations |
159 |
distributed by the Molecular Science Computing Facility, Environmental and Molecular Sciences Laboratory, which is part of the Pacific Northwest Laboratory, P.O. Box 999, Richland, Washington 99352, USA, and is funded by the U.S. Department of Energy. The Pacific Northwest Laboratory is a multiprogram laboratory operated by Battelle Memorial Institute for the U.S. Department of Energy under contract DE-AC06-76RLO 1830.
51.C. E. Moore, National Standard Reference Data Series 34 (U.S. Government Printing Office, Washington, D.C., 1970).
52. Information |
on |
G2 |
calculations |
for |
ionization |
energies |
and |
electron |
affinities |
was |
obtained |
from |
the |
following |
URL: |
http://chemistry.anl.gov/compmat/comptherm.htm |
|
|
|
||||
53.J. A. Pople, M. Head-Gordon, D. J. Fox, K. Raghavachari, and L. A. Curtiss, J. Chem. Phys. 90, 5622 (1989).
54.L. A. Curtiss, C. Jones, G. W. Trucks, K. Raghavachari, and J. A. Pople, J. Chem. Phys. 93, 2537 (1990).
55.J. Berkowitz, G. B. Ellison, and D. Guttman, J. Phys. Chem. 78, 2744 (1994).
56.H. Hotop and W. C. Lineberger, J. Phys. Chem. Ref. Data 14, 731 (1985).
57.D. W. Arnold, S. E. Bradforth, T. N. Kitsopoulos, and D. M. Neumark, J. Chem. Phys. 95, 8753 (1991).
58.B. Ruscic and J. Berkowitz, J. Chem. Phys. 95, 4033 (1991).
59.K. K. Murray, D. G. Leopold, T. M. Miller, and W. C. Lineberger, J. Chem. Phys. 89, 5442 (1988).
60.S. E. Bradforth, E. H. Kim, D. W. Arnold, and D. M. Neumark, J. Chem. Phys. 98, 800 (1993).
61.K. M. Ervin, J. Ho, and W. C. Lineberger, J. Phys. Chem. 92, 5405 (1988).
62.S. G. Lias, J. E. Bartmess, J. E. Liebman, J. L. Holmes, R. D. Levin, and W. G. Mallard, J. Phys. Chem. Ref. Data Suppl. 1, 17 (1988).
63.M. K. Gilles, M. L. Polak, and W. C. Lineberger, J. Chem. Phys. 96, 8012 (1992).
64.M. K. Gilles, K. M. Ervin, J. Ho, and W. C. Lineberger, J. Phys. Chem. 96, 1130 (1992).
65.H. W. Sarkas, J. H. Hendricks, S. T. Arnold, and K. H. Bowen, J. Chem. Phys. 100, 1884 (1994).
66.J. M. Oakes, L. B. Harding, and G. B. Ellison, J. Chem. Phys. 83, 5400 (1985).
67.Handbook of Chemistry and Physics, D. R. Lide (Ed.), CRC, Boca Raton (1996).
68.B. Ruscic and J. Berkowitz, J. Chem. Phys. 98, 2568 (1993).
69.B. Ruscic, E. H. Appelman, and J. Berkowitz, J. Chem. Phys. 95, 7957 (1991).
70.R. J. Lipert and S. D. Coulson, J. Chem. Phys. 92, 3240 (1990).
71.B. Ruscic and J. Berkowitz, J. Chem. Phys. 95, 4378 (1991).
72.J. Berkowitz, E. H. Appelman, and W. A. Chupka, J. Chem. Phys. 58, 1950 (1973).
73.B. Ruscic and J. Berkowitz, J. Chem. Phys. 95, 2407 (1991).
74.B. Ruscic and J. Berkowitz, J. Chem. Phys. 95, 2416 (1991).
75.Singlet electron affinities:  HNO, LiH,
HNO, LiH,  HCF,
HCF, 
 Singlet ionization energies: HF,
Singlet ionization energies: HF, 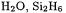 HCl,
HCl,  cyclopropene, CS,
cyclopropene, CS,

160 |
|
|
Chapter 5 |
ClF, |
thiooxirane, |
propyne, |
HOF, |
|
|
|
NCCN, CO, |
|
|
Doublet electron affinities: HCCO, HCO, |
|
HOO, NCO, |
OF, |
|
CCH, |
|
|
CN, OH, |
SH, |
Doublet ionization energies: |
|
CN, |
|
