
Учебники / Textbook and Color Atlas of Salivary Gland Pathology - DIAGNOSIS AND MANAGEMENT Carlson 2008
.pdf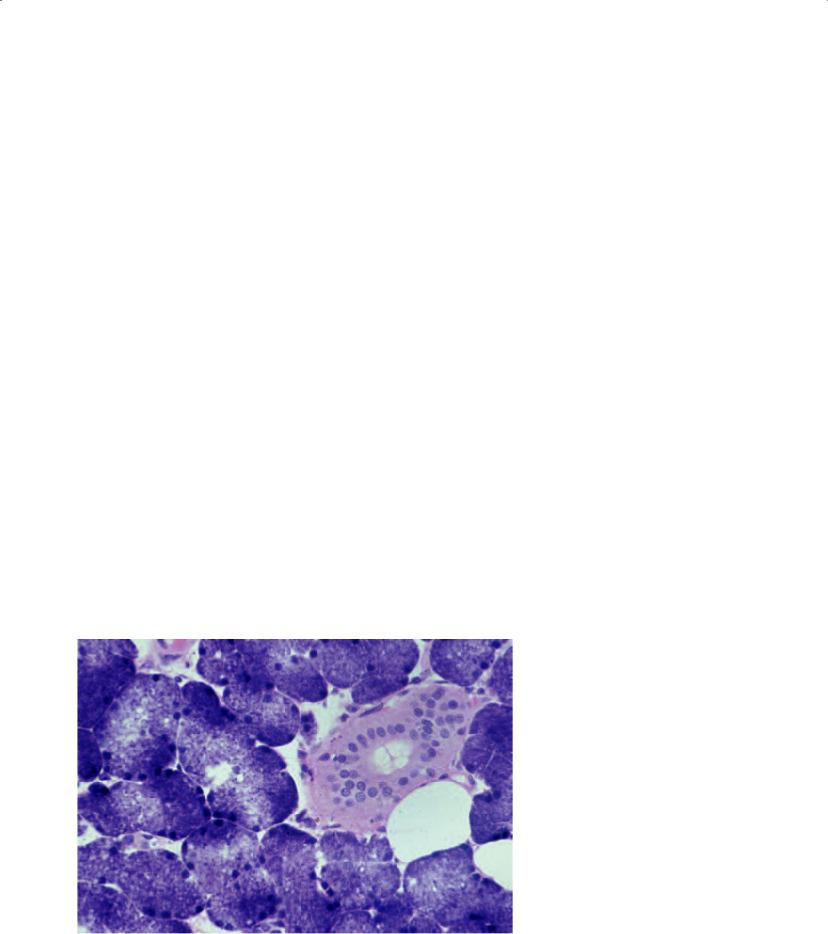
146 Systemic Diseases Affecting the Salivary Glands
cirrhosis results in asymptomatic enlargement of the parotid glands in 30–80% of these patients (Regezi, Sciubba, and Jordan 2003). In such cases, parotid enlargement has been attributed to protein deficiency. In diabetes mellitus, the mechanism of acinar hypertrophy associated with this condition is unknown. Due to the numerous causes of sialosis, as well as a large number of diagnoses that can clinically resemble sialosis, the patient’s history is paramount in such cases so as to properly initiate the diagnostic process. In addition, the treatment for these disorders differs significantly.
CLINICAL MANIFESTATIONS OF SIALOSIS
Sialosis is characterized by chronic, afebrile salivary enlargement. The enlargement is described by patients as slowly evolving and recurrent. A thorough history will most frequently divulge symptoms associated with comorbid disease such as diabetes mellitus, achalasia, alcoholism, or others.
DIAGNOSIS OF SIALOSIS WITH SALIVARY GLAND BIOPSY
The role of salivary gland biopsy in a patient suspected of having sialosis is to rule out Sjogren’s
syndrome, sarcoidosis, and lymphoma. Sialosis is a disease limited to the major salivary glands such that an incisional biopsy of parotid enlargement is indicated, rather than an incisional biopsy of the lip as might be considered in Sjogren’s syndrome or sarcoidosis. As such, a minor salivary gland biopsy is of no value in making a diagnosis of sialosis. While histopathologic confirmation of this process is valuable, it is certainly possible to make a clinical diagnosis of sialosis based on historical findings (Mandel, Vakkas, and Saqi 2005). In addition, once a histopathologic diagnosis of sialosis has been established, the underlying cause of this disorder must be ascertained, if not already known preoperatively. Prompt treatment of the underlying disease process must then occur.
Histopathology of Sialosis
The parotid swelling of sialosis is due to acinar enlargement (Figure 6.16). The diameter of the acinar cell tends to increase by two to three times that of normal. The nuclei tend to be basally situated, and the cytoplasm tends to be packed with granules. There is no correlation between the specific clinical type of sialosis and the histologic appearance. Inflammatory cells tend to be absent. The long-standing nature of the underlying disease may ultimately lead to acinar atrophy and replacement with fat (Werning 1991).
Figure 6.16. The histopathology of the incisional parotid biopsy performed on the patient in Figure 6.14. Acinar hypertrophy is noted. The physical, radiographic, and histologic information confirms a diagnosis of achalasia. He was treated accordingly.

Summary
•A number of systemic diseases may infiltrate salivary gland tissue, including Sjogren’s syndrome, sarcoidosis, and sialosis.
•Sjogren’s syndrome and sarcoidosis are autoimmune disorders, while sialosis is not.
•Sjogren’s syndrome is characterized by keratoconjunctivitis sicca and xerostomia, with or without association with another connective tissue disease.
•Approximately 30% of patients with Sjogren’s syndrome will develop salivary gland enlargement, most commonly in the parotid gland.
•A salivary gland biopsy may confirm the patient’s diagnosis of Sjogren’s syndrome. Either a labial biopsy or parotid gland biopsy may be performed. Disease may be identified more often in a parotid biopsy, even when the patient does not demonstrate parotid swelling.
•Another benefit of parotid biopsy is the identification of lymphoma that is known to develop in 5–10% of patients with Sjogren’s syndrome.
•Specific histologic criteria have been established for the diagnosis of Sjogren’s syndrome in salivary gland biopsies, specifically referred to as a focus.
•Sarcoidosis is a multisystem disease, with particular proclivity for lung involvement.
•Erythema nodosum represents cutaneous nodules most commonly involving the extensor surfaces of the lower extremities, and occurs in about two-thirds of patients with sarcoidosis.
•Lofgren’s syndrome involves a triad of hilar lymphadenopathy, erythema nodosum, and arthritis.
•Approximately 5% of patients with sarcoidosis have parotid gland enlargement.
•The triad of anterior uveitis in conjunction with parotitis and facial nerve palsy has been referred to as Heerfordt’s syndrome.
•As with Sjogren’s syndrome, there is a higher yield of positive findings to make a diagnosis of sarcoidosis based on parotid biopsy compared to lip biopsy.
•Sialosis is a non-inflammatory, non-neoplas- tic, non-autoimmune disorder showing enlargement of the salivary glands, most notably the parotid gland.
Systemic Diseases Affecting the Salivary Glands |
147 |
References
Abbondanzo SL. 2001. Extranodal marginal-zone B-cell lymphoma of the salivary gland. Ann Diag Pathol 5(4):246–254.
Anaya JM, Tobon GJ, Vega P, Castiblanco J. 2006. Autoimmune disease aggregation in families with primary Sjogren’s syndrome. J Rheumatol 33:2227–2234.
Cai YL, Wang ZH, Lu SJ. 2002. Analysis for therapy and prognosis of undifferentiated carcinoma with lymphoid stroma in the salivary gland. Shanghai Kou Qiang Yi Xue 11(4):310–313 (in Chinese).
Chisolm DM, Mason DK. 1968. Labial salivary gland biopsy in Sjogren’s syndrome. J Clin Pathol 21:656– 660.
Daniels TE. 1991. Benign lymphoepithelial lesion and Sjogren’s syndrome. In: Ellis GL, Auclair PL, Gnepp DR (eds.), Surgical Pathology of the Salivary Glands. Philadelphia: W.B. Saunders, pp. 83–106.
Daniels TE, Silverman S, Michalski JP, et al. 1975. The oral component of Sjogren’s syndrome. Oral Surg Oral Med Oral Pathol 39:875–885.
Dawson LJ, Stanbury J, Venn N et al. 2006. Antimuscarinic antibodies in primary Sjogren’s syndrome reversibly inhibit the mechanism of fluid secretion by human submandibular salivary acinar cells. Arthritis Rheum 54:1165–1173.
Del Bono V, Pretolesi F, Pontali E et al. 2000. Possible malignant transformation of benign lymphoepithelial lesions in human deficiency virus-infected patients: Report of three cases. Clin Infect Dis 30(6):947–949.
Fatahzadeh M, Rinaggio J. 2006. Diagnosis of systemic sarcoidosis prompted by orofacial manifestations: A review of the literature. JADA 137:54–60.
Folwaczny M, Sommer A, Sander CA, Kellner H. 2002. Parotid sarcoidosis mimicking Sjogren’s syndrome: Report of a case. J Oral Maxillofac Surg 60:117–120.
Garcia-Carrasco M, Fuentes-Alexandro S, Escarcega RO et al. 2006. Pathophysiology of Sjogren’s syndrome. Arch Med Res 37:921–932.
Godwin JT. 1952. Benign lymphoepithelial lesion of the parotid gland (adenolymphoma, chronic inflammation, lymphoepithelioma, lymphocytic tumor, Mikulicz disease): Report of eleven cases. Cancer 5:1089– 1103.
Greenspan JS, Daniels TE, Talal N, Sylvester RA. 1974. The histopathology of Sjogren’s syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol
37:217–229.
Hellmann DB. 1993. Sarcoidosis. In: Schumacher HR, Klippel JH, Koopman WJ (eds.), Primer on the Rheumatic Diseases (10th ed.). Atlanta, GA: Arthritis Foundation, pp. 204–205.
Ihrler S, Zietz C, Sendelhofert A et al. 1999. Lymphoepithelial duct lesions in Sjogren-type sialadenitis. Virchows Arch 434(4):315–323.

148 Systemic Diseases Affecting the Salivary Glands
Kasamatsu A, Kanazawa H, Watanabe T, Matsuzaki O. 2007. Oral sarcoidosis: Report of a case and review of literature. J Oral Maxillofac Surg 65:1256–1259.
Kulkarni K. 2005. Unusual presentation of Sjogren syndrome. Southern Med J 98:1210–1211.
Mandel L, Kaynar A. 1994. Sialadenopathy: A clinical herald of sarcoidosis. Report of two cases. J Oral Maxillofac Surg 52:1208–1210.
Mandel L, Vakkas J, Saqi A. 2005. Alcoholic (beer) sialosis.
J Oral Maxillofac Surg 63:402–405.
Marx RE. 1995. Incisional parotid biopsy for diagnosis of systemic disease. Oral Maxillofac Surg Clinics of North Am 7:505–517.
Marx RE, Hartman KS, Rethman KV. 1988. A prospective study comparing incisional labial to incisional parotid biopsies in the detection and confirmation of sarcoidosis, Sjogren’s disease, sialosis and lymphoma. J Rheumatol 15:621–629.
Morgan WS, Castleman B. 1953. A clinicopathologic study of “Mikulicz’s disease.” Am J Pathol 29:471–503.
Moutsopoulos HM. 1993. In: Schumacher HR (ed.), Primer on the Rheumatic Diseases (10th ed.) Atlanta: Arthritis Foundation, pp. 131–135.
Nessan VJ, Jacoway JR. 1979. Biopsy of minor salivary glands in the diagnosis of sarcoidosis. New Engl J Med 301:922–924.
Perez P, Kwon YJ, Alliende C et al. 2005. Increased acinar damage of salivary glands of patients with Sjogren’s syndrome is paralleled by simultaneous imbalance of matrix metalloproteinase 3/tissue inhibitor of metalloproteinases 1 and matrix metalloproteinase 9/tissue inhibitor of metalloproteinases 1 ratios. Arthritis Rheum 52:2751–2760.
Pijpe J, Kalk WWI, Bootsma H et al. 2007. Progression of salivary gland dysfunction in patients with Sjogren’s syndrome. Ann Rheum Dis 66:107–112.
Regezi JA, Sciubba JJ, Jordan RCK. 2003. In: Regezi JA, Sciubba JJ, Jordan RCK (eds.), Oral Pathology, Clinical Pathologic Correlations (4th ed.). Philadelphia: W.B. Saunders, pp. 183–217.
Talal N, Bunim J. 1964. The development of malignant lymphoma in the course of Sjogren’s syndrome. Am J Med 36:529–540.
Vairaktaris E, Vassiliou S, Yapijakis C et al. 2005. Salivary gland manifestations of sarcoidosis: Report of three cases. J Oral Maxillofac Surg 63:1016–1021.
Werning JT. 1991. Infectious and systemic diseases. In: Ellis GL, Auclair PL, Gnepp DR (eds.), Surgical Pathology of the Salivary Glands. Philadelphia: W.B. Saunders, pp. 39–59.

Chapter 7
Classification, Grading, and Staging of
Salivary Gland Tumors
John J. Sauk, DDS, MS, FAAAS, FAHNS
Outline
Introduction
Classification Systems for Salivary Gland Neoplasms The Cellular Classification of Salivary Gland
Neoplasms
Benign Salivary Gland Neoplasms Pleomorphic Adenoma
Papillary Cystadenoma Lymphomatosum Monomorphic Adenomas
Basal Cell Adenoma
Canalicular Adenoma Oncocytoma Sebaceous Adenoma
Sebaceous Lymphadenoma Myoepithelioma Cystadenoma
Ductal Papillomas Sialoblastoma
Malignant Epithelial Neoplasms
Mucoepidermoid Carcinoma
Adenoid Cystic Carcinoma Adenocarcinomas
Acinic Cell Carcinoma
Polymorphous Low-Grade Adenocarcinoma (PLGA)
Adenocarcinoma, NOS (Not Otherwise Specified) Rare Adenocarcinomas
Basal Cell Adenocarcinoma Clear Cell Carcinoma Cystadenocarcinoma Sebaceous Adenocarcinoma
Sebaceous Lymphadenocarcinoma Oncocytic Carcinoma
Salivary Duct Carcinoma
Primary Mucinous Adenocarcinoma Malignant Mixed Tumors
Carcinoma Ex-pleomorphic Adenoma Salivary Carcinosarcoma Metastasizing Mixed Tumor
Rare Carcinomas
Primary Squamous Cell Carcinoma
Epithelial-Myoepithelial Carcinoma
Anaplastic Small Cell Carcinoma
Undifferentiated Carcinomas
Small Cell Undifferentiated Carcinoma
Large Cell Undifferentiated Carcinoma
Lymphoepithelial Carcinoma
Myoepithelial Carcinoma
Adenosquamous Carcinoma
Non-epithelial Neoplasms
Lymphomas and Benign Lymphoepithelial Lesion
Mesenchymal Neoplasms
Malignant Mesenchymal Salivary Gland Tumors
Malignant Secondary Neoplasms
Grading and Staging of Salivary Gland Tumors
Molecular Systematics of Salivary Gland Neoplasms
Staging of Salivary Gland Tumors
Summary
References
Introduction
Scientific classification is a method by which scientists categorize species of organisms. Modern classification has its root in the work of Carolus Linnaeus, also known as Carl von Linné, the Father of Taxonomy, who grouped species according to shared physical characteristics. Accordingly, scientific classification belongs to the science of taxonomy or biological systematics. Molecular systematics, which uses genomic and proteomic expression data, has driven many recent revisions in these systems. In a like manner, surgeons, pathologists, and oncologists have endeavored to symmetrically categorize tumors on the basis of designated characteristics so as to predict probable biological behavior that would help dictate appropriate therapeutic modalities to be employed and to forecast a probable prognosis. As such, the “designated characteristics” upon which
149

150 Classification, Grading, and Staging of Salivary Gland Tumors
tumors have been classified may be in more contemporary terms considered as “biomarkers.” In like fashion, genomic and proteomic expression profiles of tumors have thrust pathology and oncology into molecular systematics to develop classifications.
The role of molecular profiling or systematics for clinical decision making and taxonomy has been recently considered, and the classification of tissue or other specimens for diagnostic, prognostic, and predictive purposes based on multiple gene expression and proteomics has been noted to hold major promise for optimizing the management of patients with cancer (Ioannidis 2007). However, assay development and data analysis have been principally investigative, and there exists a lofty potential for the introduction of bias. Most troubling is that standardization of profiles has been the exception. Moreover, classifier performance is typically overinterpreted by conveying the results as p-values or multiplicative effects, whereas the absolute sensitivity and specificity of classification are modest, particularly when tested in large validation samples (Ioannidis 2007). Furthermore, validation has frequently been made with less than favorable consideration for methodology and safeguarding for bias. Most disconcerting is that the postulated classifier performance can be inflated compared to what these profiles can accomplish.
Whether traditional morphological designated characteristics or molecular systematics are employed, the aim of any classification is to demonstrate diagnostic, prognostic, and predictive performance. This can generally be accomplished for any data set by training. However, it is well known that unless training is unsupervised (no knowledge of the correct class is involved), the performance of the system being tested on the training dataset is totally uninformative about its true operation (Ioannidis 2007). Cross-validation and independent validation are two methods to determine whether the proposed scheme is an accurate classifier (Allison et al. 2006). Despite the method employed, different metrics can be used to describe the classifier performance. These may include statistical testing measures, multiplicative effect measures such as likelihood ratios or hazard ratios, or absolute effect measures. Although all information has some value, absolute effect measures (sensitivity and specificity) are the most meaningful from a clinical perspective (Buyse et al. 2006).
Classification Systems for Salivary Gland Neoplasms
The classification system for salivary gland neoplasms has evolved with the accumulation of clinical experience and our understanding of the basis for neoplasia. Although a variety of classifications have been advocated, there has been some regional variation in terminology and classification between European and American authors. Historically, the first and most notable classification was that put forth by Foote and Frazell (1954). Later systems reflect the recognition and description of previously unrecognized entities or the deletion of some terms that were misnomers or were considered meaningless. The succeeding classifications include those by Thackray and Lucas (1974); Evans and Cruickshank (1970); the World Health Organization (WHO); Thackray and Sobin (1972); Batsakis (1979); Seifert et al. (1986); and Ellis and Auclair (Auclair, Ellis, and Gnepp 1991). The most recent fascicle on the subject provides a stepwise evolution of these taxonomies (Ellis and Auclair 1996).
THE CELLULAR CLASSIFICATION OF SALIVARY GLAND NEOPLASMS
Salivary gland neoplasms are noted for their histological variability. Reflecting the anatomy of these glands, benign and malignant salivary gland neoplasms may arise either from epithelial, mesenchymal, or lymphoid origins. The complexity of the classification and the rarity of some of these tumors, some of which display a wide spectrum of morphological patterns within the same tumor and the existence of hybrid lesions, challenge the surgical pathologist with a difficult task in differentiating benign from malignant tumors (Seifert and Donath 1996; Speight and Barrett 2002).
The following cellular classification system reiterates that advocated by the National Cancer Institute (NCI) of the U.S. Public Health Service (USPHS) (www.cancer.gov), which is derived heavily from that published by the Armed Forces Institute of Pathology (AFIP) (Ellis and Auclair 1996) (Table 7.1). Similar to the NCI scheme we also include malignant non-epithelial neoplasms because these lesions embrace a sizable proportion of salivary gland neoplasms. Though less common,

Classification, Grading, and Staging of Salivary Gland Tumors |
151 |
Table 7.1. Cellular classification of salivary gland tumors.
Epithelial neoplasms
Benign salivary gland neoplasms Pleomorphic adenoma or mixed tumor
Papillary cystadenoma lymphomatosum or Warthin’s tumor
Monomorphic adenomas Basal cell adenoma Canalicular adenoma Oncocytoma Sebaceous adenoma
Sebaceous lymphadenoma Myoepithelioma Cystadenoma
Ductal papillomas Sialoblastoma
Malignant epithelial neoplasms Mucoepidermoid carcinoma Adenoid cystic carcinoma Adenocarcinomas
Acinic cell carcinoma
Polymorphous low-grade adenocarcinoma Adenocarcinoma, NOS
Rare adenocarcinomas Basal cell adenocarcinoma Clear cell carcinoma Cystadenocarcinoma
Sebaceous adenocarcinoma Sebaceous lymphadenocarcinoma Oncocytic carcinoma
Salivary duct carcinoma
Primary mucinous adenocarcinoma Malignant mixed tumors
Carcinoma ex-pleomorphic adenoma Salivary carcinosarcoma Metastasizing mixed tumor
Rare carcinomas
Primary squamous cell carcinoma Epithelial-myoepithelial carcinoma Anaplastic small cell carcinoma Undifferentiated carcinomas
Small cell undifferentiated carcinoma Large cell undifferentiated carcinoma Lymphoepithelial carcinoma
Myoepithelial carcinoma Adenosquamous carcinoma
Non-epithelial neoplasms
Lymphomas and benign lymphoepithelial lesion Mesenchymal neoplasms
Malignant mesenchymal Salivary gland tumors
the inclusion of malignant secondary tumors is presented to be inclusive.
As noted in the introduction, statistics regarding incidence, frequency, and prognosis have varied depending on the study. Moreover, a cursory review of the literature generally reveals that power analyses are rarely performed to access the necessary sample size for many of these studies, and most rudimentary statistical measures are employed to arrive at conclusions. In large part, these deficiencies have stemmed from the general rare incidence of many salivary gland neoplasms. Although the AFIP statistics have been criticized to be biased because of the methods of case accrual as a reference service, these data are probably the most reliable, especially for rare and unusual lesions.
Benign Salivary Gland Neoplasms
Pleomorphic Adenoma
The pleomorphic adenoma or mixed tumor is the most common salivary gland tumor, representing 45–75% of all salivary gland tumors. Clinically, the tumors are smooth, multilobular, and appear encapsulated. However, microscopically, tumor extensions may be seen extending beyond the apparent capsule. The tumor is varied depending on the cellularity and the myxoid content. The presence of both epithelial and mesenchymal-like elements resulting from epithelial cells and myoepithelial cells produces significant diversity in the appearance of these tumors. Notable is that stromal components may encompass myxoid, fibroid, or chondroid features providing the mixed appearance of these lesions (Ellis and Auclair 1996).
Papillary Cystadenoma Lymphomatosum
The papillary cystadenoma lymphomatosum or Warthin’s tumor has been regarded as the second most common benign salivary gland neoplasm, and comprises 6–10% of all parotid tumors. These tumors seldom arise in the submandibular or minor salivary glands. Men are more commonly affected than women, with a gender ratio of 5 : 1. Interestingly, the prevalence increases in smokers and bilateral distribution has been noted in 12% of cases (Ellis and Auclair 1996).
Monomorphic Adenomas
Basal Cell Adenoma
The basal cell adenoma is a lesion characterized by a monomorphous uniform basiloid pattern and

152 Classification, Grading, and Staging of Salivary Gland Tumors
lacking any myxochondroid features typical of mixed tumors.
Canalicular Adenoma
Canalicular adenomas are benign salivary gland tumors composed of interconnecting and branching cords of columnar cells manifesting as single or double rows of cells.
Sialoblastoma
Sialoblastoma is a rare neoplasm of major salivary glands. The tumors are either congenital or arise in the prenatal period. It has been suggested that these tumors be divided into benign and malignant lesions based on cytologic features and patterns of growth that include nerve and vascular invasion and necrosis (Batsakis and Frankenthaler 1992; Ellis and Auclair 1996).
Oncocytoma
The oncocytoma is a rare benign salivary gland neoplasm composed of large eosinophilic granular cells (oncocytes) containing atypical mitochondria.
Sebaceous Adenoma
The sebaceous adenoma is a rare benign salivary gland neoplasm that demonstrates sebaceous differentiation.
Sebaceous Lymphadenoma
Sebaceous lymphadenoma is believed by some to be a variant of sebaceous adenoma but contains sebaceous glands that are surrounded by lymphoid elements.
Myoepithelioma
Myoepitheliomas are tumors that demonstrate myoepithelial differentiation and are believed to represent a spectrum of mixed tumors but which lack chondroid and myxochondroid features.
Cystadenoma
The cystadenoma is a rare benign tumor that is characterized by unicystic or polycystic growths that contain regions of overgrowth that may at times be papillary in character.
Ductal Papillomas
Ductal papillomas have been categorized by the WHO into three morphological types. These include
(a) intraductal papillomas, which are luminal papillary lesions that result in cystic dilatation of a duct; (b) inverted duct papillomas, a papillary proliferation that occurs at the junction of salivary duct and mucosal surface; and (c) sialadenoma papilliferum, an exophytic growth involving the mucosal surface and salivary ductal structures.
Malignant Epithelial Neoplasms
Mucoepidermoid Carcinoma
There still exists some conjecture about whether mucoepidermoid carcinoma exists as only lowgrade and high-grade neoplasms (Spiro et al. 1978). The presence of mucoepidermoid carcinoma is usually asymptomatic and presenting as solitary, painless masses. When present, symptoms encompass pain, drainage from the ipsilateral ear, dysphagia, trismus, and facial paralysis (Ellis and Auclair 1996). On rare occasions, mucoepidermoid carcinoma may occur within the mandible or maxilla (3 : 1) (Brookstone and Huvos 1992). These tumors are referred to as “central mucoepidermoid carcinomas” (Ellis and Auclair 1996).
Mucoepidermoid carcinomas may consist of various proportions of mucous, epidermoid, intermediate, columnar, and clear cells, and are often cystic in pattern. These tumors constitute the majority of malignant neoplasms found in both major and minor salivary glands (Speight and Barrett 2002). Thus, mucoepidermoid carcinoma embodies 29% to 34% of malignant salivary gland tumors residing in both major and minor salivary glands (Ellis and Auclair 1996; Eveson and Cawson 1985; Spiro et al. 1978; Spitz and Batsakis 1984). The best evidence to date indicates that 84–93% of these neoplasms initiate within the parotid glands (Goode, Auclair, and Ellis 1998; Guzzo et al. 2002). Among the minor salivary glands, mucoepidermoid carcinoma has an affinity for the lower lip (Ellis and Auclair 1996). Generally, the mean age for these carcinomas is 47 years; however, there exists a broad age range of 8 years to 92 years, and this is one of the few salivary gland malignancies occurring in childhood (Ellis and Auclair 1996). Notably, previous exposure to ionizing radiation has been suggested to significantly increase the risk of mucoepidermoid carcinomas of the major salivary glands (Ellis and Auclair 1996; Guzzo et al. 2002).

Classification, Grading, and Staging of Salivary Gland Tumors |
153 |
The level of microscopic grading of mucoepidermoid is of paramount importance to establish the prognosis (Auclair, Goode, and Ellis 1992; Goode, Auclair, and Ellis 1998; Speight and Barrett 2002). These tumors may be graded as low grade, intermediate grade, or high grade. The grade of a lesion is ascertained as a sum of five parameters. Sums that are 0–4 are regarded as low grade, those that are 5–6 are considered intermediate grade, and sums 7–14 are regarded as high-grade cancers. The five parameters considered are
1.Intracystic component (+2).
2.Neural invasion present (+2).
3.Necrosis present (+3).
4.Mitosis (≥4 per 10 high-power field (+3).
5.Anaplasia present (+4).
Importantly, retrospective reviews of mucoepidermoid carcinoma of the major salivary glands have revealed a statistical correlation between this point-based grading system and outcome for parotid tumors; however, similar rigor was not deemed useful for tumors of the submandibular glands (Goode, Auclair, and Ellis 1998) or minor salivary glands (Guzzo et al. 2002). When more emphasis on features of tumor invasion was employed, better correlations were obtained, indicating that tumor staging may be a better indicator of prognosis (Ellis and Auclair 1996). These results reinforce the need for analyses that encompass absolute effect measures (Brandwein et al. 2001).
Adenoid Cystic Carcinoma
Adenoid cystic carcinoma, in earlier times known as “cylindroma,” is a slow-growing but nevertheless aggressive neoplasm with a notable capacity for recurrence (Brookstone and Huvos 1992). Three growth patterns have been acknowledged: cribriform, tubular, and a solid (basaloid) pattern. The tumors are classified according to the predominant pattern (Batsakis, Luna, and el-Naggar 1990; Brookstone and Huvos 1992; Ellis and Auclair 1996). The cribriform pattern is the most common, while the solid pattern is the least common (Perzin, Gullane, and Clairmont 1978). Solid adenoid cystic carcinoma is a high-grade lesion with reported recurrence rates of as much as 100%, compared with 50–80% for the tubular and cribriform variants (Ellis and Auclair 1996).
Reports from the AFIP indicate that adenoid cystic carcinoma is the fifth most common malignant epithelial tumor of the salivary glands after mucoepidermoid carcinomas; adenocarcinomas, NOS (not otherwise specified); acinic cell carcinomas; and polymorphous low-grade adenocarcinomas (PLGAs)(Ellis and Auclair 1996). The peak incidence for this tumor is stated to be in the fourth through sixth decades of life (Ellis and Auclair 1996).
These neoplasms characteristically progress as slow-growing lesions in the preauricular or submandibular region. Not infrequently, pain and facial paralysis are noted with progressive growth of the tumors, which may be attributed to their propensity to invade nerves (Ellis and Auclair 1996). Adenoid cystic carcinomas have a tendency to have an extended course resulting in a poor clinical result. The 10-year survival for these tumors is considered to be less than 50% (Speight and Barrett 2002). In that these carcinomas characteristically repeatedly recur and spur late distant metastases, clinical staging of these lesions appears to be a more superior prognostic indicator than histologic grade (Friedrich and Bleckmann 2003; Hamper et al. 1990; Speight and Barrett 2002.)
Adenocarcinomas
Acinic Cell Carcinoma
The acinic cell carcinoma, also referred to as “acinic cell adenocarcinoma,” is a malignant epithelial neoplasm in which the tumor cells convey acinar serous differentiation as opposed to mucous acinar cells (Ellis and Auclair 1996). AFIP data has indicated that acinic cell carcinoma is the third most common salivary gland epithelial neoplasm after mucoepidermoid carcinoma and adenocarcinoma, NOS. Moreover, acinic cell carcinoma comprised 17% of primary malignant salivary gland tumors with more than 80% occurring in the parotid gland. Women are generally more affected than males, with a mean age of 44 years. However, others have reported a 0–19% frequency of acinic cell carcinoma among malignant salivary gland neoplasms (Ellis and Auclair 1996). Patients usually present with a slowly increasing mass in the parotid. Pain is a symptom in approximately 33% of patients. Again, staging appears to be a better prognostic predictor than histologic grading (Ellis and Auclair 1996).

154 Classification, Grading, and Staging of Salivary Gland Tumors
Polymorphous Low-Grade Adenocarcinoma (PLGA)
Polymorphous low-grade adenocarcinoma is a malignant tumor that is predominately restricted to minor salivary glands. The tumors are distinguished by bland, uniform nuclear features; varied but distinctive architecture; invasive growth; and perineural infiltration (Ellis and Auclair 1996). PLGAs have been reported to represent 11% of all tumors of salivary glands and 26% of malignant neoplasms. PLGAs characteristically emerge as solid, nontender swellings of the mucosa of the hard and soft palates, buccal mucosa, or upper lip. Soreness, hemorrhage, telangiectasia, or ulceration have been associated with these lesions (Ellis and Auclair 1996). These tumors are slowly progressive salivary gland neoplasms with an apparent survival approaching 80% at 25 years (Evans and Luna 2000). Noteworthy is that since some of these tumors may behave capriciously, the qualifying term “low-grade” may be deceptive and the term “polymorphous adenocarcinoma” is preferable (Speight and Barrett 2002).
The average age of patients has been 59 years, with the vast majority of cases occurring between the ages of 50 and 79 years. The gender predisposition is in favor of females in a ratio of2 : 1. Among minor gland tumors, PLGA is two times as frequent as adenoid cystic carcinoma (Ellis and Auclair 1996). The AFIP series indicates that >60% of PLGAs occur in the mucosa of either the soft or hard palates, the next most frequent sites being buccal mucosa (16%) and the upper lip (12%).
Adenocarcinoma, NOS (Not Otherwise Specified)
Adenocarcinoma, NOS demonstrates glandular or ductal differentiation but does not have any of the distinct morphologic features that typify the other, more explicit carcinoma types. The diagnosis of adenocarcinoma, NOS is fundamentally one of elimination. Adenocarcinoma, NOS has been suggested to be only second to mucoepidermoid carcinoma in frequency among malignant salivary gland neoplasms (Ellis and Auclair 1996). However, reports have shown a varied incidence from 4% to 10% (Speight and Barrett 2002). The AFIP reports the mean patient age of 58 years with roughly 40% and 60% of tumors occurring in the major and minor salivary glands, correspondingly,
with 90% of tumors occurring in the parotid gland (Ellis and Auclair 1996). Adenocarcinoma, NOS is graded according to the degree of differentiation as low-grade, intermediate-grade, and high-grade tumors (Ellis and Auclair 1996; Speight and Barrett 2002). Some reports have indicated that survival is more superior for patients with tumors of the oral cavity than for those with tumors of the major glands (Ellis and Auclair 1996; Matsuba et al. 1988).
Rare Adenocarcinomas
Basal Cell Adenocarcinoma
Basal cell adenocarcinoma, also known as basaloid salivary carcinoma, carcinoma ex monomorphic adenoma, malignant basal cell adenoma, malignant basal cell tumor, and basal cell carcinoma, is an epithelial neoplasm that is cytologically similar to basal cell adenoma but is infiltrative and has a small potential for metastasis (Ellis and Auclair 1996). In AFIP case files spanning almost 11 years, basal cell carcinoma comprised 1.6% of all salivary gland neoplasms and 2.9% of salivary gland malignancies (Ellis and Auclair 1996). Nearly 90% of tumors occurred in the parotid gland (Muller and Barnes 1996). The average age of patients is reported to be 60 years (Ellis and Auclair 1996).
Similar to most salivary gland neoplasms, swelling is typically the only sign or symptom experienced (Muller and Barnes 1996). A sudden increase in size may occur in a few patients (Ellis and Auclair 1996). Basal cell carcinomas are lowgrade carcinomas that are infiltrative, locally destructive, and tend to recur. They occasionally metastasize. In a retrospective series of 29 cases, there were recurrences in 7 and metastases in 3 (Muller and Barnes 1996). In another retrospective review of 72 cases, 37% involved local recurrences (Ellis and Auclair 1996). The overall prognosis for patients with this tumor is good (Ellis and Auclair 1996; Muller and Barnes 1996).
Clear Cell Carcinoma
Clear cell carcinoma, also known as clear cell adenocarcinoma, is a very rare, malignant epithelial neoplasm composed of a monomorphous population of cells that have optically clear cytoplasm with standard hematoxylin and eosin stains and that lack features of other specific neoplasms. Because of inconsistencies in the methods of reporting salivary gland neoplasms, meaningful

Classification, Grading, and Staging of Salivary Gland Tumors |
155 |
incidence rates for this tumor are difficult to derive from the literature (Ellis and Auclair 1996). Most cases involve the minor salivary glands. In the AFIP case files, the mean age of patients is approximately 58 years (Ellis and Auclair 1996).
In most patients, swelling is the only symptom. Clear cell adenocarcinoma is a low-grade neoplasm. As of 1996, the AFIP reported that no patient is known to have died as a result of this tumor (Ellis and Auclair 1996).
Cystadenocarcinoma
Cystadenocarcinoma, also known as malignant papillary cystadenoma, mucus-producing adenopapillary, or nonepidermoid, carcinoma, low-grade papillary adenocarcinoma of the palate, and papillary adenocarcinoma, is a rare, malignant epithelial tumor characterized histologically by prominent cystic and frequently papillary growth but lacking features that characterize cystic variants of several more common salivary gland neoplasms. Cystadenocarcinoma is the malignant counterpart of cystadenoma (Ellis and Auclair 1996).
In a review of 57 cases, the AFIP found that men and women are affected equally; the average patient age was about 59 years; and about 65% occurred in the major salivary glands, and primarily in the parotid. Most patients present with a slowly growing asymptomatic mass. Clinically, this neoplasm is rarely associated with pain or facial paralysis. Cystadenocarcinoma is considered to be a low-grade neoplasm (Ellis and Auclair 1996).
Sebaceous Adenocarcinoma
Sebaceous adenocarcinoma is an uncommon malignant epithelial neoplasm that is generally regarded as an intermediate grade neoplasm. The tumors have been noted to be comprised of islands and sheets of cells with areas of sebaceous differentiation. The cells of these tumors possess atypical nuclear morphology and manifest an infiltrative pattern of growth (Ellis and Auclair 1996). Clinical presentation as a painless, slow-growing, asymptomatic swelling has been reported; however, lesions may be painful or result in facial nerve involvement with paralysis. Approximately onethird of these tumors have been reported to have a recurrence potential (Gnepp 1983). The vast majority of examples of this neoplasm have been limited to the parotid gland with the mean age of
occurrence being 69 years (Ellis and Auclair 1996; Gnepp 1983).
Sebaceous Lymphadenocarcinoma
Sebaceous lymphadenocarcinoma is a particularly uncommon malignant low-grade neoplasm with a good prognosis (Gnepp and Brannon 1984). These neoplasms are believed to correspond to carcinomatous transformation of sebaceous lymphadenoma. The carcinoma portion of the tumor has been reported as sebaceous adenocarcinoma, however, other forms of salivary gland carcinomas have been recognized (Ellis and Auclair 1996). In that merely 3 cases, all associated with the parotid gland in elderly patients, have been reported, there is little information on these neoplasms (Ellis and Auclair 1996; Gnepp and Brannon 1984).
Oncocytic Carcinoma
Oncocytic carcinoma, or oncocytic adenocarcinoma, is a rare high-grade carcinoma, salivary neoplasm with predominantly oncocytic features. The oncocytic carcinomas constitute <1% of the cases of salivary gland tumors accessioned to the AFIP files (Ellis and Auclair 1996). The majority of reported cases have been in the parotid gland, where they present as painful lesions or are associated with paralysis (Sugimoto et al. 1993). Similar to other parotid gland carcinomas, tumors that are less than 2 cm have a better prognosis than larger tumors. Thus, TNM (T = tumor size, N = number of nodes, M = metastasis) staging correlates with the prognosis (Goode, Auclair, and Ellis 1998). The AFIP series reports that the average age of patients with these neoplasms has been 63 years (Ellis and Auclair 1996).
Salivary Duct Carcinoma
Salivary duct carcinoma, or salivary duct adenocarcinoma, is a high-grade malignant epithelial neoplasm comprised of elements that bear a resemblance to expanded salivary gland ducts. The AFIP files indicate that salivary duct carcinomas represent only 0.2% of all epithelial salivary gland neoplasms, with 85% of cases affecting the parotid gland and with a male gender predominance of 75% and mean incidence occurring in the seventh and eighth decades of life (Ellis and Auclair 1996). Parotid swelling has generally been the most common presenting symptom. However, facial
