
Cycloaddition Reactions in Organic Synthesis
.pdf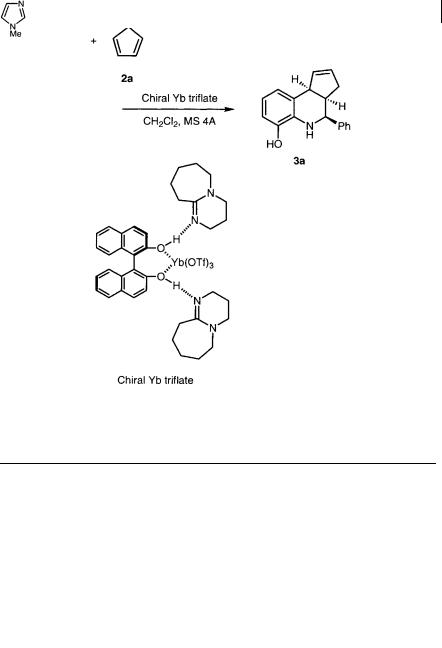
5.2 Aza Diels-Alder Reactions of Azadienes 189
Scheme 5.2 Catalytic enantioselective aza Diels-Alder reaction (1)
Tab. 5.1 Effect of additives in the asymmetric aza Diels-Alder reaction
Entry |
Additive |
Temp. ( C) |
Yield (%) |
cis/trans |
ee (%) (cis) |
|
|
|
|
|
|
1 |
– |
0 |
71 |
98 : 2 |
62 |
2 |
– |
–15 to 0 |
48 |
99 : 1 |
68 |
3 |
|
–15 to 0 |
21 |
98 : 2 |
91 |
|
(20 mol%) |
|
|
|
|
4 |
|
–15 |
92 |
>99 : 1 |
71 |
|
4a |
|
|
|
|
|
(100 mol%) |
|
|
|
|
|
|
|
|
|
|

190 5 Catalytic Enantioselective Aza Diels-Alder Reactions
tylpyridine (DTBP, 4b) gave the best result in the reaction of imine 1a with ethyl vinyl ether (2b), whereas higher selectivity was obtained when DTBMP (4a) or 2,6- diphenylpyridine (DPP, 4c) was used in the reaction of imine 1b with 2b. This could be explained by the slight difference in the asymmetric environment created by Yb(OTf)3, (R)-(+)-BINOL, DBU, and the additive. While use of butyl vinyl ether (2c) reduced selectivity (entry 7), dihydrofuran (2d) reacted smoothly to achieve high selectivity (entries 8 and 9). It was found that the imine (1c) prepared from cyclohexanecarboxaldehyde and 2-hydroxyaniline was unstable and difficult to purify. The asymmetric aza Diels-Alder reaction was successfully per-
Tab. 5.2 Catalytic enantioselective aza Diels-Alder reactions using azadienes
Entry |
Imine |
Alkene |
Product |
Additive |
Yield (%) |
cis/trans |
ee (%) (cis) |
|
|
|
|
|
|
|
|
|
|
1 a) |
1a |
2b |
3a |
4b |
58 |
94 |
: 6 |
61 |
2 a, b) |
1a |
2b |
3a |
4b |
52 |
94 |
: 6 |
77 |
3 |
1b |
2b |
3b |
4b |
69 |
> 99 : < 1 |
86 |
|
4 |
1b |
2b |
3b |
4c |
65 |
99 |
: 1 |
91 |
5 |
1b |
2b |
3b |
4a |
74 |
> 99 : < 1 |
91 |
|
6 b) |
1b |
2b |
3b |
4a |
62 |
98 |
: 2 |
82 |
7 |
1b |
2c |
3c |
4a |
80 |
66 |
: 34 |
70 |
8 |
1b |
2d |
3d |
4a |
90 |
91 |
: 9 |
78 |
9 |
1b |
2d |
3d |
4c |
67 |
93 |
: 7 |
86 |
10 c) |
1c |
2a |
3e |
4a |
58 |
> 99 : < 1 |
73 |
|
|
|
|
|
|
|
|
|
|
a)The reaction was performed at –45 C
b)10 mol% of the chiral Yb triflate was used
c)Chiral Sc catalyst was used
5.3 Aza Diels-Alder Reactions of Azadienophiles 191
formed using the three-component-coupling procedure (successively addition of the aldehyde, the amine, and cyclopentadiene) in the presence of Sc(OTf) [6d, 7a, 10] (instead of Yb(OTf)3), (R)-(+)-BINOL, DBU, and DTBMP (4a).
The assumed transition state of this reaction is shown in Scheme 5.3. Yb(OTf)3, (R)-(+)-BINOL, and DBU form a complex with two hydrogen bonds, and the axial chirality of (R)-(+)-BINOL is transferred via the hydrogen bonds to the amine parts. The additive would interact with the phenolic hydrogen of the imine, which is fixed by bidentate coordination to Yb(III). Because the top face of the imine is shielded by the amine, the dienophiles approach from the bottom face to achieve high levels of selectivity.
Scheme 5.3 Assumed transition state (triflate ions are omitted for clarity)
(i)Asymmetric aza Diels-Alder reactions between achiral azadienes and dienophiles have been achieved using a catalytic amount of a chiral source.
(ii)The unique reaction pathway in which the chiral Lewis acid activates not dienophiles but dienes, has been revealed. In most asymmetric Diels-Alder reactions reported using chiral Lewis acids, the Lewis acids activate dienophiles [11].
(iii)A unique lanthanide complex including an azadiene and an additive, which is quite different from the conventional chiral Lewis acids, has been developed.
5.3
Aza Diels-Alder Reactions of Azadienophiles
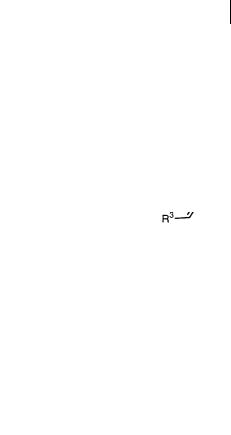
While the above results have demonstrated the catalytic enantioselective aza DielsAlder reactions of azadienes, the catalytic enantioselective aza Diels-Alder reactions of azadienophiles were reported using a chiral zirconium compound.
Chiral zirconium compound 6 [12] was prepared from Zr(Ot-Bu)4, 2 equiv. (R)- 6,6 -Br-1,1 -binaphthol ((R)-Br-BINOL, 5) [13], and 2–3 equiv. of a ligand

192 5 Catalytic Enantioselective Aza Diels-Alder Reactions
(Scheme 5.4), and the model reaction of the imine derived from 1-naphthaldehyde and 2-aminophenol (1b) with Danishefsky’s diene (7a) [14] was investigated. It was found that ligands and solvents strongly influenced the yields and enantioselectivity (Table 5.3). The importance to the selectivity of the free hydroxyl group of the aldimine was already known [4, 12]. Actually, when the imine prepared from aniline or 2-methoxyaniline was used under the same reaction conditions, the corresponding pyridone derivatives were obtained in good yields but low ee (aniline, 68% yield, 4% ee; 2-methoxyaniline, 74% yield, 25% ee). For ligands, N- methylimidazole (NMI) gave the best result. When the chiral catalyst (10 mol%) was prepared in dichloromethane, the desired aza Diels-Alder reaction of aldimine 1b with diene (7a) proceeded smoothly, but the enantiomeric excess of the adduct was only 40%. On the other hand, the enantioselectivity was improved to 61% ee when the catalyst was prepared in benzene by stirring at room temperature for 1 h, the mixture was evaporated to remove benzene and t-BuOH under reduced pressure, and the reaction was then performed in dichloromethane. Although use of a bulky diene (4-t-butoxy-2-trimethylsiloxy-1,3-butadiene, 7b) [15] reduced the selectivity in this instance, a higher enantiomeric excess was obtained when the catalyst was prepared in toluene. The best result was finally obtained when the preparation of the catalyst and the successive reaction was carried out in toluene (without removing the solvent) [16].
Scheme 5.4 Preparation of chiral zirconium catalyst (6)
The effect of the metals used was then examined (Table 5.4). When the group 4 metals, titanium, zirconium, and hafnium, were screened it was found that a chiral hafnium catalyst gave high yields and enantioselectivity in the model reaction of aldimine 1b with 7a, while lower yields and enantiomeric excesses were obtained using a chiral titanium catalyst [17].
Several examples of catalytic aza Diels-Alder reactions using the chiral zirconium catalyst are shown in Table 5.5 [18]. High chemical yields and good to high
5.3 Aza Diels-Alder Reactions of Azadienophiles 193
Tab. 5.3 Catalytic enantioselective aza Diels-Alder reactions using imino dienophiles (1)
Entry |
Additive (mol%) |
Solvent |
Yield (%) |
ee (%) |
||
|
|
|
|
|
|
|
1 |
NMI (30) |
CH2Cl2 |
74 |
40 |
||
2 |
NMI (30) |
C6H6 –CH2Cl2 |
81 |
61 |
||
|
|
|
|
|
(83 |
41) a) |
3 |
NMI (30) |
Toluene –CH2Cl2 |
81 |
71 |
||
4 |
NMI (30) |
Toluene |
86 |
82 |
||
5 |
DMI (20) |
Toluene |
76 |
59 |
||
6 |
|
Toluene |
28 |
24 |
||
|
|
(30) |
||||
7 |
(30) |
Toluene |
86 |
50 |
||
8 |
(30) |
Toluene |
81 |
46 |
||
9 |
– |
Toluene |
65 |
10 b) |
||
a)4-t-Butoxy-2-trimethylsilyloxy-1,3-butadiene (7b) was used
b)Reverse enantioselectivity was observed
enantioselectivity was usually obtained in the presence of 5–20 mol% of the chiral catalyst. 4-Methoxyl-3-methyl-2-trimethylsiloxy-1,3-butadiene (7c) [19] also worked well under standard conditions and the desired 2,3-dihydro-4-pyridone derivatives were obtained in high yields with high enantioselectivity. As for the R1 group in Table 5.5, ortho-substituted aromatic compounds gave higher selectivity. For example, although the imine derived from benzaldehyde (1a) reacted with 7c to afford the corresponding adduct in 65% ee (Table 5.5, entry 10), 77% ee of the pyridone derivative was obtained in the reaction of the imine derived from o-tolualdehyde (1d) with 7c under the same reaction conditions (entry 9). The imine derived

1945 Catalytic Enantioselective Aza Diels-Alder Reactions
Tab. 5.4 Effect of metals
Entry |
M (catalyst) |
mol% |
Yield (%) |
ee (%) |
|
|
|
|
|
1 |
Zr (6) |
10 |
86 |
82 |
2 |
Zr (6) |
20 |
96 |
88 |
3 |
Hf |
10 |
89 |
73 |
4 |
Hf |
20 |
96 |
84 |
5 |
Ti |
10 |
68 |
39 |
6 |
Ti |
20 |
70 |
62 |
|
|
|
|
|
Tab. 5.5 Catalytic enantioselective aza Diels-Alder reactions using imino dienophiles (2)
Entry |
R1 |
Diene |
Amount of |
Yield (%) |
ee (%) |
|
|
|
5 (mmol) |
|
|
|
|
|
|
|
|
1 |
1-Nap (1b) |
7a |
5 |
72 |
67 |
2 |
|
|
10 |
86 |
82 |
3 |
|
|
20 |
96 |
88 |
4 |
|
7c |
10 |
79 |
89 |
5 |
|
|
20 |
93 |
93 |
6 |
|
7a |
10 |
92 |
80 |
7 |
o-Me-Ph (1d) |
7a |
10 |
81 |
76 |
8 |
|
|
20 |
83 |
82 |
9 |
|
7c |
20 |
97 |
77 |
10 |
Ph (1a) |
7c |
20 |
83 |
65 |
11 |
|
7a |
10 |
86 |
64 |
12 |
c-C6H11 (1c) |
7c |
10 |
47 |
78 a) |
13 |
|
|
20 |
51 |
86 a) |
a) The imine was prepared from cyclohexanecarboxaldehyde and 2-amino-3-methylphenol

5.4 A Switch of Enantiofacial Selectivity 195
from 2-thiophenecarboxyaldehyde reacted with 7a smoothly to give the corresponding pyridone derivative in high yield and good enantiomeric excess. In the reaction of the imine derived from cyclohexanecarboxaldehyde (1c) with 7c, low enantiomeric excess of the adduct was observed under standard reaction conditions. The low selectivity was attributed to isomerization of the cis and trans conformation of the aldimine. To prevent the isomerization, the imine derived from cyclohexanecarboxaldehyde and 2-amino-3-methylphenol was used. As expected, the enantiomeric excess of the corresponding pyridone derivative was improved to 86% ee.
5.4
A Switch of Enantiofacial Selectivity
Synthesis of both enantiomers is a very important task not only in organic chemistry but also in medicinal and bioorganic chemistry [20]. In chemical transformations, syntheses of both enantiomers are generally performed using both enantiomers of chiral sources. Although there are many chiral sources in nature, it is sometimes difficult to obtain both enantiomers, for example those of amino acids, monosaccharides, alkaloids, etc. When such chiral sources are employed in asymmetric syntheses, preparation of both enantiomers is difficult. Mechanistically, on the other hand, both enantiomers can be produced by controlling the enantiofaces of prochiral compounds. It should, therefore, be possible to control them by designing ligands which even have the same chirality [21, 22].
On the basis of this consideration the catalyst-substrate interaction, including the reaction course in chiral zirconium-catalyzed aza Diels-Alder reactions, was examined. It was indicated from both experiments and modeling studies that the substituents at the 3,3 -positions of the BINOL ligand strongly influenced enantioselectivity [23]. In the presence of Zr(Ot-Bu)4 (20 mol%), (R)-6,6 -dibromo-3,3 - diphenyl-1,1 -binaphthol (8a, 40 mol%), and NMI (60 mol%), imine 1d, prepared from o-tolualdehyde and 2-aminophenol, reacted with Danishefsky’s diene (7a) in toluene at –45 C to afford the corresponding piperidine derivative in 84% ee (Table 5.6, entry 1). The absolute configuration was proved to be (R), which was the reverse of that using (R)-Br-BINOL (5) instead of 8a under the same reaction conditions (entry 2). Several reaction conditions were examined and interesting effects of molecular sieves (MS) were found. When the reaction was conducted at 0 C without molecular sieves the enantioselectivity decreased to 57% ee (entry 3). In contrast, 90% ee of the product was obtained when MS 3 Å was added to the reaction pot (entry 4). Although MS 4 Å and 5 Å were also effective, the enantioselectivity decreased when the reaction was performed at –45 C with MS 3 Å (entries 5–7). Furthermore, the best result was obtained when the reaction was performed at 23 C in benzene (entries 8 and 9). It is noted that the chemical yield was also improved by more than 25% and that 91% ee of the product was obtained even at 23 C. When 10 mol% of the catalyst was used, chemical yield and enantioselectivity decreased (81%, 77% ee).

1965 Catalytic Enantioselective Aza Diels-Alder Reactions
Tab. 5.6 Effect of solvents, temperature, and molecular sieves
Entry |
Solvent |
Temp. ( C) |
MS |
Yield (%) |
ee (%) |
|
|
|
|
|
|
1 |
Toluene |
–45 |
None |
66 |
84 |
2 |
Toluene |
–45 |
None |
83 |
82 (S) a) |
3 |
Toluene |
0 |
None |
45 |
57 |
4 |
Toluene |
0 |
MS 3 Å |
80 |
90 |
5 |
Toluene |
0 |
MS 4 Å |
76 |
89 |
6 |
Toluene |
0 |
MS 5 Å |
77 |
89 |
7 |
Toluene |
–45 |
MS 3 Å |
54 |
77 |
8 |
Toluene |
23 |
MS 3 Å |
96 |
88 |
9 |
Benzene |
23 |
MS 3 Å |
93 |
91 |
a) Catalyst 6 was used
Chart 5.1
Other examples were tested and the results are summarized in Table 5.7 [24]. The reactions always proceeded smoothly to afford the corresponding piperidine derivatives in high yields with high enantiomeric excess. In addition, reverse enantio-

5.4 A Switch of Enantiofacial Selectivity 197
Tab. 5.7 Catalytic asymmetric aza Diels-Alder reactions
Entry |
R1 |
R2 |
Yield (%) |
ee (%) |
|
|
|
|
|
1 |
Ph (1a) |
H |
94 |
82 |
2 |
Ph (1a) |
Me |
Quant. |
80 |
3 |
o-MePh (1d) |
H |
93 |
91 |
4 |
o-MePh (1d) |
Me |
98 |
89 |
5 |
-Nap (1b) |
H |
88 |
84 |
6 |
-Nap (1b) |
Me |
78 |
80 |
7 |
|
H |
67 |
85 |
8 |
|
Me |
78 |
87 |
9 |
|
H |
90 |
90 |
10 |
|
Me |
87 |
88 |
11 |
2-thiophene |
H |
74 |
86 a) |
12 |
2-thiophene |
Me |
70 |
86 a) |
13 |
c-C6H11 (1c) |
H |
64 |
81 b) |
14 |
c-C6H11 (1c) |
Me |
67 |
80 b) |
|
|
|
|
|
a)10 was used instead of 8a
b)The imine was prepared from cyclohexanecarboxaldehyde and 2-amino-3-methylphenol
selectivity was observed in these reactions compared with those obtained using 6 as a catalyst. It is noteworthy that chemical yields and enantiomeric excesses were usually improved by using the new catalyst system.
The precise structure of the zirconium catalyst was examined by NMR analysis. When Zr(Ot-Bu)4 (1 equiv.), 8b (2 equiv.), and NMI (3 equiv.) were combined in benzene-d6 at 23 C, two independent species which were assigned to a new zirconium catalyst and free 8b were observed. Although the signals of free 8b were still observed when Zr(Ot-Bu)4 (1 equiv.), 8b (1 equiv.), and NMI (3 equiv.) were stirred at 23 C, only the signals assigned to the new zirconium catalyst were detected when the mixture was stirred at 80 C for 2.5 h. These results indicated the formation of 9b as the new zirconium catalyst. The structure was also supported by an experiment in which Zr(Ot-Bu)4 (0.2 equiv.), 8a (0.2 equiv.), NMI (0.6 equiv.), and MS 3 Å were combined in benzene and the mixture was stirred for 2.5 h at 80 C (formation of 9a). Imine 1d (1 equiv.) and 7a (1.2 equiv.) were then added to the catalyst solution, and the mixture was stirred for 48 h at 23 C. After the same work-up procedures as described above, the desired piperidine derivative was obtained in > 98% yield with 89% ee, values comparable with those
198 5 Catalytic Enantioselective Aza Diels-Alder Reactions
obtained when the catalyst was prepared by combining Zr(Ot-Bu)4 (1 equiv.), 8a (2 equiv.), and NMI (3 equiv.) at 23 C (Table 5.7, entry 3). It was assumed that formation of 9 was slow and incomplete at 23 C, because of the bulky 3,3 -phenyl groups of 8.
The assumed transition state for this reaction is shown in Scheme 5.5. The two bulky t-butoxy groups are expected to locate at the two apical positions. One of the 3,3 -phenyl groups would effectively shield one face of an imine, and consequently, a diene attacks from the opposite side. Judging from this model, similar selectivities were expected in the Mannich-type reactions of imines with silyl enolates. Actually, when ligand 10 was used in the reaction of imine 1a with S-ethyl- thio-1-trimethylsiloxyethene, the corresponding -amino thioester was obtained in 84% ee (Scheme 5.6). As expected, the sense of the chiral induction in this case was the reverse of that observed when using catalyst 6 [12, 25].
Scheme 5.5 Assumed transition state
5.5
Chiral Catalyst Optimization
Although high yields and selectivity were sometimes attained in chiral zirconiumcatalyzed aza Diels-Alder reactions, further optimization of the catalyst structure was desired. In addition, another problem was the rather high loading of the catalyst (10–20 mol%). To address these issues, optimization of the catalyst structure was planned using both solid-phase and liquid-phase methods. Solid-phase and liquidphase methods have advantages and disadvantages, and it is believed that combining the advantages of these methods leads to the most efficient catalyst optimization.
