
Cycloaddition Reactions in Organic Synthesis
.pdf
78 2 Recent Advances in Palladium-catalyzed Cycloadditions involving Trimethylenemethane and its Analogs
Scheme 2.27 TMM [4+3] cycloadditions with 1,3-dienes
[3+2] cycloaddition tends to interfere more with the [4+3] cycloaddition when the diene acceptor is not restricted in the cisoid form. The Pd-catalyzed reaction of vinylcyclopentenes (111) with the parent TMM gave both fiveand seven-mem- bered ring adducts with ratio depending on the steric nature of the substrate. While the ketone (111, X = O) yielded only a [3+2] adduct, derivatization of the carbonyl with bulky ketal substituents does serve to enhance seven-membered ring formation, presumably because of a combination of both steric and electronic effect (Scheme 2.30) [41]. In contrast to the pyrone system, addition of Me3SnOAc does not seem to impact the ratio of the cycloaddition products except for some rate enhancement [42].
The [4+3] cycloaddition has also been realized in acceptors containing a nitrogen atom. While , -unsaturated aldimines, and structurally flexible ketimine such as (87), generally only undergo [3+2] cycloadditions (see Scheme 24), the ketimine (112), which is rigidly held in a cisoid conformation, does give exclusively the [4+3] adduct azepine (113). On the other hand, the steroidal imine (114) produces a quantitative yield of a 1 : 1 mixture of the [4+3] and [3+2] cycloadducts (115) and (116), respectively (Scheme 2.31) [36].
2.4 Application in Organic Synthesis 79
Scheme 2.28 TMM [4+2] cycloadditions with pyrones
Scheme 2.29 TMM [4+3] cycloadditions in the synthetic study of pseudolaric acid B (106)

80 2 Recent Advances in Palladium-catalyzed Cycloadditions involving Trimethylenemethane and its Analogs
Scheme 2.30 TMM [4+3] cycloadditions with structurally flexible 1,3-dienes
Scheme 2.31 TMM [4+3] cycloadditions with aza-1,3-dienes
2.4.9
[6+3] Cycloadditions
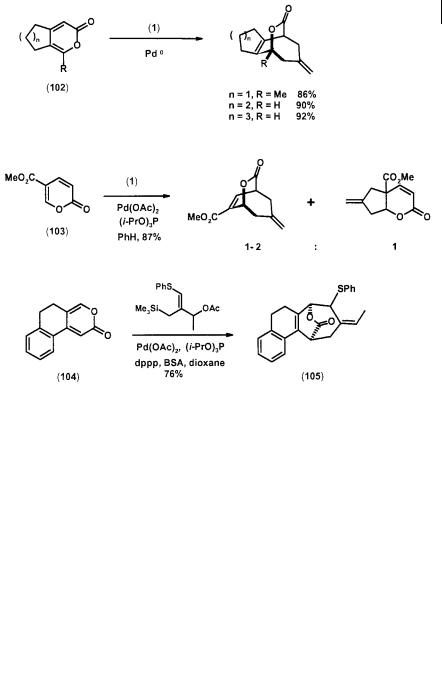
Nine-membered ring systems are potentially accessible via a TMM cycloaddition with conjugated trienes in a [6+3] fashion. However, tropone is the only reported system that undergoes such a reaction. Interestingly, these cycloadditions are remarkably selective in that only nine-membered ring products are formed,
2.4 Application in Organic Synthesis 81
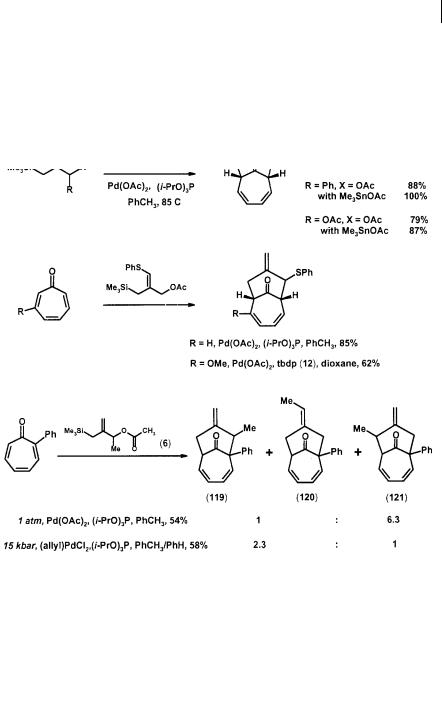
although [3+2] and [4+3] modes of cycloaddition are also possible (Scheme 2.32). The parent TMM precursor (1) reacts with tropones (117) to give reasonable yields of the bridged [4.3.1]decanones (118) [43]. Various substituted TMMs also cycloadd to tropone with regioselectivity similar to that of the corresponding [3+2] cases [20, 43]. Addition of Me3SnOAc as a co-catalyst also leads to yield improvement [16]. In the case of a phenyl-substituted tropone and a methyl-TMM, performing the reaction under high pressure favors the formation of kinetic products (119) and (120) over the thermodynamic product (121) [11].
Scheme 2.32 TMM [6+3] cycloadditions with tropones

82 2 Recent Advances in Palladium-catalyzed Cycloadditions involving Trimethylenemethane and its Analogs
Carboxylative TMM cycloaddition has also been realized with 3-methoxytropone and precursor (56) to produce an epimeric mixture of acids (122), which was employed in a synthetic study of the bicyclic diterpene sanadaol (123). The use of bidentate ligand tpdp (12) and high pressure did not improve the reaction. However, the addition of Me3SnOAc as a co-catalyst did produce a better yield of (122) (Scheme 2.33) [16].
Scheme 2.33 Carboxylative TMM [4+3] cycloadditions with tropone in the synthetic study of sanadaol (123)
2.4.10
[3+3] Cycloaddition
There is only one report in the literature of a [3+3] cycloaddition involving TMM and activated aziridines to give the corresponding piperidine (124) [44]. The formation of the six-membered ring adduct is presumed to proceed via the ringopening of the aziridine by the attack of TMM complex (2) on the least hindered carbon, which is then followed by an intramolecular cyclization (Scheme 2.34).
Scheme 2.34 TMM [3+3] cycloaddition with aziridines

References 83
2.5
Conclusions
The chemistry of palladium-catalyzed TMM cycloadditions has indeed come a long way since its invention. The effort of Trost and others has succeeded in transforming a laboratory curiosity into a most powerful synthetic method. This versatile methodology has steadily gained in popularity over the years in the synthetic community in the area of cyclopentanoid construction. As presented in this review, much progress has been made in expanding the scope and fine-tuning the chemistry. Factors, such as the acyl and tin effects and high pressure, to control the reactivity and selectivity have been discovered. Sevenand nine-membered rings are now also accessible with this chemistry. With these new developments, TMM cycloadditions are becoming standard tools in fiveand possibly larger membered ring construction, much like the Diels-Alder reaction in six-membered ring synthesis.
References
[1](a) B. M. Trost, D. M. T. Chan, J. Am. Chem. Soc. 1979, 101, 6429–6432; (b) B.
M.Trost, D. M. T. Chan, ibid 1979, 101, 6432–6433.
[2](a) B. M. Trost, Angew. Chem., Int. Ed. Engl. 1986, 25, 1–20, and references cited therein; (b) B. M. Trost, Pure Appl. Chem. 1988, 60, 1615–1626.
[3]Y. Inoue, H. Hashimoto, Senryo to Yakuhin 1985, 30, 3109–3123.
[4]D. M. T. Chan, in Comprehensive Organic Synthesis, Vol. 5 (Eds. B. M. Trost, I. Fleming, L. A. Paquette), Pergamon, Oxford, 1991, pp 271–314.
[5]M. Lautens, W. Klute, W. Tam, Chem. Rev. 1996, 96, 49–62.
[6]The formation of TMM complex from Group VIII transition metal such as Ir, Ru, and Os from precursors derived from (1) has been reported: M. D. Jones,
R.D. W. Kemmitt, J. Chem. Soc., Chem. Commun., 1985, 811–812.
[7]C.-C. Su, J.-T. Chen, G.-H. Lee, Y. Wang, J. Am. Chem. Soc. 1994, 116, 4999–5000.
[8]D. J. Gordon, R. F. Fenske, T. N. Nanninga, B. M. Trost, J. Am. Chem. Soc.
1981, 103, 5974–5975.
[9]It is interesting to note that a recent report of a kinetic isotope effects study of cycloaddition of (1) with a nearly symme-
trical fumarate acceptor strongly implicates a concerted mechanism in that system: D. A. Singleton, B. E. Schulmeier,
J.Am. Chem. Soc. 1999, 121, 9313–9317.
[10]B. M. Trost, D. M. T. Chan, J. Am. Chem. Soc. 1983, 105, 2326–2335.
[11]B. M. Trost, J. R. Parquette, A. L. Marquart, J. Am. Chem. Soc. 1995, 117, 3284–3285.
[12]L. A. Paquette, D. R. Sauer, D. G. Cleary, M. A. Kinsella, C. M. Blackwell, L. G. Anderson, J. Am. Chem. Soc.
1992, 114, 7375–7387.
[13]B. M. Trost, D. M. T. Chan, J. Am. Chem. Soc. 1983, 105, 2315–2325.
[14]S. Raghavan, M. Ishida, H. Shinozaki,
K.Nakanishi, Y. Ohfune, Tetrahedron Lett. 1993, 34, 5765–5768.
[15]C. Holzapfel, T. L. van der Merwe, Tetrahedron Lett. 1996, 37, 2307–2310.
[16]B. M. Trost, J. H. Haugan, unpublished results.
[17]L. L. Shiu, T. I. Lin, S. M. Peng, G. R. Her, D. D. Ju, S. K. Lin, J. H. Hwang,
C.Y. Mou, T. Y. Luh, J. Chem. Soc.,
Chem. Commun. 1994, 5, 647–648.
[18]B. M. Trost, J. R. Parquette, C. Nubling, Tetrahedron Lett. 1995, 36, 2917– 2920.
[19]B. M. Trost, J. R. Parquette, J. Org. Chem. 1994, 59, 7568–7569.

84 2 Recent Advances in Palladium-catalyzed Cycloadditions involving Trimethylenemethane and its Analogs
[20]B. M. Trost, M. C. Matelich, J. Am. Chem. Soc. 1991, 113, 9007–9009.
[21]B. M. Trost, R. I. Higuchi, J. Am. Chem. Soc. 1996, 118, 10094–10105.
[22]B. M. Trost, T. A. Grese, J. Org. Chem.
1992, 57, 686–697.
[23]T. A. Grese, Ph. D. thesis, Stanford Univ., Stanford, CA, USA, 1991.
[24]B. M. Trost, T. A. Grese, D. M. T. Chan,
J. Am. Chem. Soc. 1991, 113, 7350–7362.
[25]B. M. Trost, T. A. Grese, J. Am. Chem. Soc. 1991, 113, 7363–7372.
[26]D. M. T. Chan in Encyclopedia of Reagents for Organic Synthesis, Vol. 1 (Ed. L. A. Paquette), John Wiley & Sons, England, 1995, pp 787–790.
[27]B. M. Trost, S. M. Mignani, T. N. Nanninga, J. Am. Chem. Soc. 1988, 110, 1602–1608.
[28]B. M. Trost, S. M. Mignani, T. N. Nanninga, J. Am. Chem. Soc. 1986, 108, 6051–6053.
[29]B. M. Trost, P. Seoane, S. Mignani, M. Acemoglu, J. Am. Chem. Soc. 1989, 111, 7487–7500.
[30]D. M. T. Chan, Ph. D. thesis, Univ. of Wisconsin–Madison, WI, USA, 1982.
[31]B. M. Trost, S. A. King, T. Schmidt, J. Am. Chem. Soc. 1989, 111, 5902–5915.
[32]B. M. Trost, S. A. King, J. Am. Chem. Soc. 1990, 112, 408–476.
[33]B. M. Trost, P. J. Bonk, J. Am. Chem. Soc. 1985, 107, 8277–8279.
[34]No successful TMM cycloaddition to acetylenes has ever been reported.
[35]M. D. Jones, R. D. W. Kemmitt, J. Chem. Soc., Chem. Commun. 1986, 1201–1203.
[36]B. M. Trost, C. M. Marrs, J. Am. Chem. Soc. 1993, 115, 6636–6645.
[37]B. M. Trost, T. N. Nanninga, D. M. T. Chan, Organometallics, 1982, 1, 1543– 1545.
[38]B. M. Trost, D. T. MacPherson, J. Am. Chem. Soc. 1987, 109, 3483–3484.
[39]B. M. Trost, S. Schneider, Angew. Chem. Int. Ed. Engl. 1989, 28, 213–215.
[40]R. I. Higuchi, Ph. D. thesis, Stanford Univ., Stanford, CA, USA, 1995.
[41]B. M. Trost, S. Yamazaki, Chem. Lett.
1994, 12, 2245–2248.
[42]B. M. Trost, S. Harmsen, unpublished results.
[43]B. M. Trost, P. Seoane, J. Am. Chem. Soc. 1987, 109, 615–617.
[44]R. B. Bambal, R. D. W. Kemmitt, J. Organomet. Chem. 1989, 362, C18–C20.

Cycloaddition Reactions in Organic Synthesis. 85
Edited by S. Kobayashi and K. A. Jorgensen
Copyright © 2001 Wiley-VCH Verlag GmbH
ISBNs: 3-527-30159-3 (Hardcover); 3-527-60025-6 (Electronic)
3
Enantioselective [2+1] Cycloaddition: Cyclopropanation with Zinc Carbenoids
Scott E. Denmark and Gregory Beutner
3.1
Introduction
Catalytic, enantioselective cyclopropanation enjoys the unique distinction of being the first example of asymmetric catalysis with a transition metal complex. The landmark 1966 report by Nozaki et al. [1] of decomposition of ethyl diazoacetate 3 with a chiral copper (II) salicylamine complex 1 (Scheme 3.1) in the presence of styrene gave birth to a field of endeavor which still today represents one of the major enterprises in chemistry. In view of the enormous growth in the field of asymmetric catalysis over the past four decades, it is somewhat ironic that significant advances in cyclopropanation have only emerged in the past ten years.
Scheme 3.1
From a historical perspective it is interesting to note that the Nozaki experiment was, in fact, a mechanistic probe to establish the intermediacy of a copper carbenoid complex rather than an attempt to make enantiopure compounds for synthetic purposes. To achieve synthetically useful selectivities would require an extensive exploration of metals, ligands and reaction conditions along with a deeper understanding of the reaction mechanism. Modern methods for asymmetric cyclopropanation now encompass the use of countless metal complexes [2], but for the most part, the importance of diazoacetates as the carbenoid precursors still dominates the design of new catalytic systems. Highly effective catalysts developed in
86 3 Enantioselective [2+1] Cycloaddition: Cyclopropanation with Zinc Carbenoids
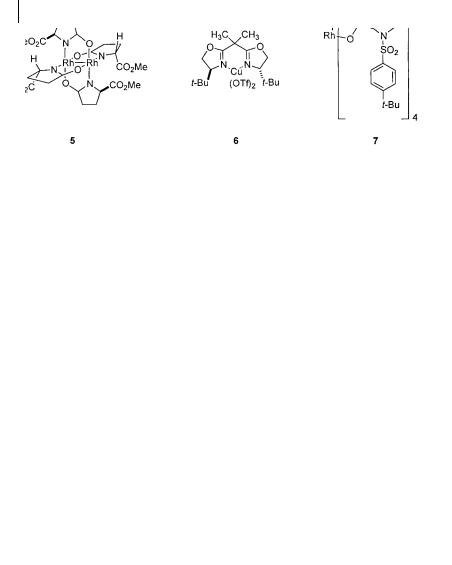
Fig. 3.1 Cyclopropanation catalysts employing diazoacetate precursors
the laboratories of Doyle (5) [3 a, b], Evans (6) [3 c] and Davies (7) [3 d, e] have shown that diazoacetates can serve as versatile precursors in highly enantioand diastereoselective cyclopropanations (Fig. 3.1).
Whereas the utility of these methods has been amply documented, they are limited in the structures they can provide because of their dependence on the diazoacetate functionality and its unique chemical properties. Transfer of a simple, unsubstituted methylene would allow access to a more general subset of chiral cyclopropanes. However, attempts to utilize simple diazo compounds, such as diazomethane, have never approached the high selectivities observed with the related diazoacetates (Scheme 3.2) [4]. Traditional strategies involving rhodium [3 a, c], copper [ 3b, 5] and palladium have yet to provide a solution to this synthetic problem. The most promising results to date involve the use of zinc carbenoids albeit with selectivities less than those obtained using the diazoacetates.
Scheme 3.2
This review outlines developments in zinc-mediated cyclopropanation from the initial reports in the 1950s through to the current state of the art methods. The presentation will rely heavily on how the evolution of mechanistic understanding aided in the rationalization and optimization of each new advance in the asymmetric process.
The most logical starting point is a discussion of the structure of the zinc carbenoid, followed by a somewhat chronological presentation of major advances in the use of zinc carbenoids in cyclopropanation. After a brief historical recounting of Simmons and Smith’s original studies, the crucial implications of diastereose-

3.2 The Simmons-Smith Cyclopropanation – Historical Background 87
lective, substrate-directed reactions will be discussed. The main body of the review involves the examination of the various types of enantioselective processes. Stereocontrol in Simmons-Smith cyclopropanations has been achieved by a number of strategies and these will be presented in the progression of:
(i)auxiliary-based methods;
(ii)chirally-modified reagents; and
(iii)chiral, catalytic processes.
The discussion of the catalytic, asymmetric variants will incorporate a significant emphasis on the interplay of mechanistic investigations and synthetic optimization studies to provide a unified picture of the cyclopropanation methods. Finally, recent insights provided by computational analysis of the transition structures for cyclopropanation will be discussed.
The primary objective of this review is to provide an integrated analysis of the contributions from structural, mechanistic and preparative studies toward the successful development of a modern, stereoselective reaction. The synthetic aspects of this useful transformation have been extensively reviewed elsewhere [6].
3.2
The Simmons-Smith Cyclopropanation – Historical Background
Without question, the most powerful method for cyclopropane formation by methylene transfer is the well-known Simmons-Smith reaction [6]. In 1958, Simmons and Smith reported that the action of a zinc–copper couple on diiodomethane generates a species that can transform a wide variety of alkenes into the corresponding cyclopropanes (Scheme 3.3) [7].
Scheme 3.3
