
Cycloaddition Reactions in Organic Synthesis
.pdf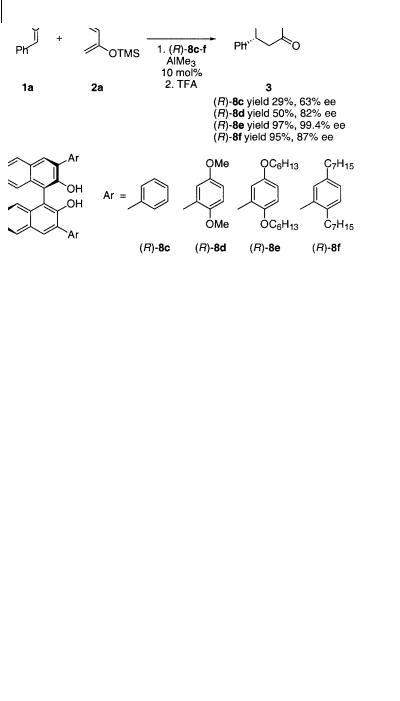
158 4 Catalytic Enantioselective Cycloaddition Reactions of Carbonyl Compounds
Scheme 4.7
atoms from the BINOL ligand and the methyl substituent are located in the equatorial plane with one of the ligand hypercoordinating ether oxygen atoms and the benzaldehyde oxygen atom as the two axial ligands, as shown in Scheme 4.8 [13]. The 2,5-dimethoxyphenyl substituent which is not coordinating to aluminum is oriented perpendicular to the BINOL ligand, whereas the 2,5-dimethoxyphenyl substituent which hypercoordinates to aluminum is twisted towards the metal. This twisting creates a chiral environment as the non-hypercoordinated 2,5-di- methoxyphenyl substituent shields the si face of benzaldehyde while the re face is available for approach by the diene.
Scheme 4.8

4.3 Cycloaddition Reactions of Carbonyl Compounds 159
The mechanism of the cycloaddition reaction of benzaldehyde 2a with Danishefsky’s diene 3a catalyzed by aluminum complexes has been investigated theoretically using semi-empirical calculations [14]. It was found that the reaction proceeds as a step-wise cycloaddition reaction with the first step being a nucleophiliclike attack of Danishefsky’s diene 2a on the coordinated carbonyl compound leading to an aldol-like intermediate which is stabilized by interaction of the cation with the oxygen atom of the Lewis acid. The next step is the ring-closure step, giving the cycloaddition product.
A series of chiral binaphthyl ligands in combination with AlMe3 has been used for the cycloaddition reaction of enamide aldehydes with Danishefsky’s diene for the enantioselective synthesis of a chiral amino dihydroxy molecule [15]. The cycloaddition reaction, which was found to proceed via a Mukaiyama aldol condensation followed by a cyclization, gives the cycloaddition product in up to 60% yield and 78% ee.
It should also be noted that chiral menthoxydichloroaluminum complexes can catalyze the cycloaddition reaction of carbonyl compounds but only small ee have been obtained [16].
Chiral boron(III) Lewis acid catalysts have also been used for enantioselective cycloaddition reactions of carbonyl compounds [17]. The chiral acyloxylborane catalysts 9a–9d, which are also efficient catalysts for asymmetric Diels-Alder reactions [17, 18], can also catalyze highly enantioselective cycloaddition reactions of aldehydes with activated dienes. The arylboron catalysts 9b–9c which are airand moisture-stable have been shown by Yamamoto et al. to induce excellent chiral induction in the cycloaddition reaction between, e.g., benzaldehyde and Danishefsky’s dienes such as 2b with up to 95% yield and 97% ee of the cycloaddition product cis-3b (Scheme 4.9) [17].
Scheme 4.9

160 4 Catalytic Enantioselective Cycloaddition Reactions of Carbonyl Compounds
The cycloaddition reaction catalyzed by complex 9d proceeds well for several aromatic and , -unsaturated aldehydes and has been used in an enantioselective route to the carbon-branched pyranose derivative cis-3c (Scheme 4.10) [17].
Scheme 4.10
A series of chiral boron catalysts prepared from, e.g., N-sulfonyl -amino acids has also been developed and used in a variety of cycloaddition reactions [18]. Corey et al. have applied the chiral (S)-tryptophan-derived oxazaborolidine-boron catalyst 11 and used it for the conversion of, e.g., benzaldehyde 1a to the cycloaddition product 3a by reaction with Danishefsky’s diene 2a [18 h]. This reaction 1a affords mainly the Mukaiyama aldol product 10, which, after isolation, was converted to 3a by treatment with TFA (Scheme 4.11). It was observed that no cycloaddition product was produced in the initial step, providing evidence for the two-step process.
Scheme 4.11
4.3.1.2 Chiral Transitionand Lanthanide-metal Complexes
Different chiral transitionand lanthanide-metal complexes can catalyze the cycloaddition reaction of unactivated and activated aldehydes with especially activated

4.3 Cycloaddition Reactions of Carbonyl Compounds 161
dienes. For the chiral titanium catalysts the focus has been on the use of mainly BINOL-titanium(IV) complexes for cycloaddition reactions. These catalysts have been widely used as chiral catalysts in enantioselective C–C bond-forming reactions of aldehydes [8, 19].
Keck et al. reported that a catalyst generated from (S)- or (R)-BINOL 12 and Ti(O-i-Pr)4 in a 2 : 1 ratio is more selective than the catalyst formed from a 1 : 1 mixture [19 f ]. The former catalyst was shown to catalyze the cycloaddition reaction of aldehydes 1 with Danishefsky’s diene 2a affording the dihydropyrones 3 with moderate to excellent enantioselectivity (Scheme 4.12). The reaction proceeds well for different aldehydes with up to 97% ee and good yield of the cycloaddition products.
Scheme 4.12
The dihydropyrones are not produced directly in the initial BINOL-titanium(IV)-cat- alyzed reaction. The major product at this stage is the Mukaiyama aldol product which is subsequently cyclized by treatment with TFA [19f ]. The formal cycloaddition product 3d (97% ee) obtained from -(benzyloxy)acetaldehyde is an important intermediate for compactin and mevinolin. Scheme 4.13 outlines how the structural subunit 13 is available in three steps via this cycloaddition approach [19 f ].
Scheme 4.13

162 4 Catalytic Enantioselective Cycloaddition Reactions of Carbonyl Compounds
The chiral BINOL/Ti(O-i-Pr)4 combination has also been used as a very enantioselective catalyst for the cyclocondensation of polyfluoroalkylaldehydes with Danishefsky’s diene leading to polyfluoroalkyldihydropyrenones in moderate yields (35–60%) and with high ee (90–98.5% ee) [20].
A chiral vanadium complex, bis(3-(heptafluorobutyryl)camphorato)oxovana- dium(IV), can catalyze the cycloaddition reaction of, mainly, benzaldehyde with dienes of the Danishefsky type with moderate to good enantioselectivity [21]. A thorough investigation was performed with benzaldehyde and different activated dienes, and reactions involving double stereo differentiation using a chiral aldehyde.
Chiral salen chromium and cobalt complexes have been shown by Jacobsen et al. to catalyze an enantioselective cycloaddition reaction of carbonyl compounds with dienes [22]. The cycloaddition reaction of different aldehydes 1 containing aromatic, aliphatic, and conjugated substituents with Danishefsky’s diene 2a catalyzed by the chiral salen-chromium(III) complexes 14a,b proceeds in up to 98% yield and with moderate to high ee (Scheme 4.14). It was found that the presence of oven-dried powdered 4 Å molecular sieves led to increased yield and enantioselectivity. The lowest ee (62% ee, catalyst 14b) was obtained for hexanal and the highest (93% ee, catalyst 14a) was obtained for cyclohexyl aldehyde. The mechanism of the cycloaddition reaction was investigated in terms of a traditional cycloaddition, or formation of the cycloaddition product via a Mukaiyama aldol-reaction path. In the presence of the chiral salen–chromium(III) catalyst system 1H NMR spectroscopy of the crude reaction mixture of the reaction of benzaldehyde with Danishefsky’s diene revealed the exclusive presence of the cycloaddition-pathway product. The Mukaiyama aldol condensation product was prepared independently and subjected to the conditions of the chiral salen-chromium(III)-catalyzed reactions. No detectable cycloaddition product could be observed. These results point towards a [2 + 4]-cycloaddition mechanism.
Scheme 4.14

4.3 Cycloaddition Reactions of Carbonyl Compounds 163
Jacobsen et al. took an important step towards the development of a more general catalytic enantioselective cycloaddition reaction of carbonyl compounds by introducing chiral tridentate Schiff base chromium(III) complexes 15 (Scheme 4.15) [23]. These complexes are highly diastereoand enantioselective catalysts for the reaction of unactivated aldehydes and can catalyze the reaction of less nucleophilic dienes. The adamantyl-substituted catalysts 15a,b gave the best results and both aliphatic and aromatic aldehydes underwent cycloaddition reactions; it was found that the use of catalyst 15b resulted in a faster and more enantioselective reaction and that the reaction can proceed in the absence of solvent. The reaction was tested for a variety of dienes, e.g. 1-methoxy-1,3-butadiene reacts with TBSOCH2CHO in the presence of only 0.5 mol% catalyst 15a to give the corresponding cycloaddition product in > 99% ee. This latter reaction provides, after hydrolysis and oxidation to the corresponding lactone, efficient access to interesting natural product structures.
Scheme 4.15
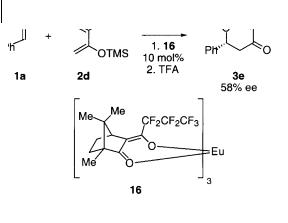
Danishefsky et al. were probably the first to observe that lanthanide complexes can catalyze the cycloaddition reaction of aldehydes with activated dienes [24]. The reaction of benzaldehyde 1a with activated conjugated dienes such as 2d was found to be catalyzed by Eu(hfc)3 16 giving up to 58% ee (Scheme 4.16). The ee of the cycloaddition products for other substrates was in the range 20–40% with 1 mol% loading of 16. Catalyst 16 has also been used for diastereoselective cycloaddition reactions using chiral O-menthoxy-activated dienes derived from (–)- menthol, giving up to 84% de [24 b, c]; it has also been used for the synthesis of optically pure saccharides.
It has been shown that ytterbium tris-(R)-(–)-1,1 -binaphthyl-2,2 -diyl phosphonate can catalyze the reaction of aromatic aldehydes with Danishefsky’s diene to
164 4 Catalytic Enantioselective Cycloaddition Reactions of Carbonyl Compounds
Scheme 4.16
give the cycloaddition product in good yield and with up to 93% ee [25]. It was found that 2,6-lutidine can improve the catalytic properties.
4.3.2
Reactions of Activated Aldehydes
Different main-group-, transitionand lanthanide-metal complexes can catalyze the cycloaddition reaction of activated aldehydes with activated and non-activated dienes. The chiral metal complexes which can catalyze these reactions include complexes which enable substrates to coordinate in a monoor bidentate fashion.
4.3.2.1 Chiral Aluminum and Boron Complexes
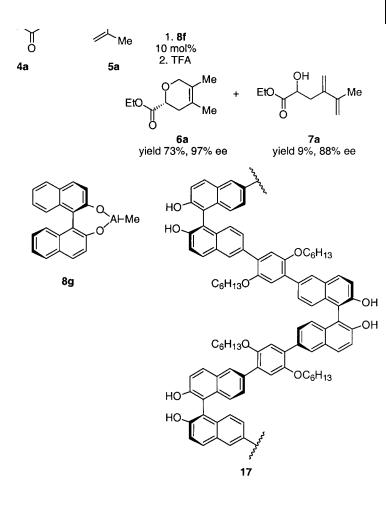
Chiral BINOL-AlMe complexes can catalyze a highly chemoand enantioselective cycloaddition reaction of activated aldehydes with conjugated dienes [10]. The reaction of ethyl glyoxylate 4a with, e.g., 2,3-dimethyl-1,3-butadiene 5a, in the presence of (R)-BINOL-AlMe 8g as the catalyst, gives 6a as the major product in 73% yield with up to 97% ee and 7% of the ene product 7a (Scheme 4.17). Chiral polymer Lewis acid complexes can also be used as catalysts for the reaction in Scheme 4.17 [26]. The use of the chiral polybinaphthyl polymer 17 in combination with AlMe3 in the reaction of 4a with 5a gave 6a in 67% yield and up to 95% ee. The most important aspect of the using 17 is that it can easily be recovered by simple filtration and reused without significant change in yield or chemoor enantioselectivity.
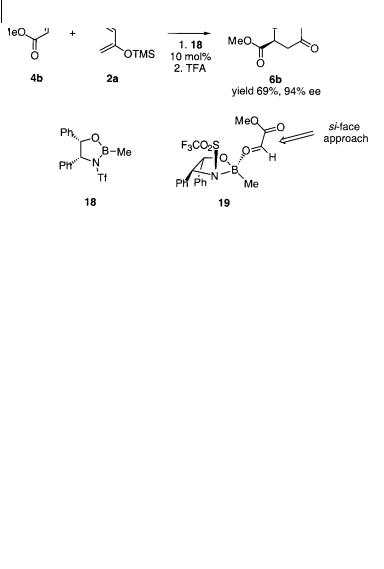
Chiral boron(III) complexes can catalyze the cycloaddition reaction of glyoxylates with Danishefsky’s diene (Scheme 4.18) [27]. Two classes of chiral boron catalyst were tested, the -amino alcohol-derived complex 18 and bis-sulfonamide complexes. The former catalyst gave the best results for the reaction of methyl glyoxylate 4b with diene 2a; the cycloaddition product 6b was isolated in 69% yield and 94% ee, while the chiral bis-sulfonamide boron complex resulted in only
4.3 Cycloaddition Reactions of Carbonyl Compounds 165
Scheme 4.17
62% ee. On the basis of the absolute configuration of 6b it was postulated that 4b coordinates in a monodentate fashion to 18 as outlined in 19, in which the re face of the activated carbonyl functionality is shielded by the triflate group, enabling the diene to approach in an endo fashion to the re face of the carbonyl functionality [27].
The interest in chiral titanium(IV) complexes as catalysts for reactions of carbonyl compounds has, e.g., been the application of BINOL-titanium(IV) complexes for ene reactions [8, 19]. In the field of catalytic enantioselective cycloaddition reactions, methyl glyoxylate 4b reacts with isoprene 5b catalyzed by BINOL-TiX2 20 to give the cycloaddition product 6c and the ene product 7b in 1 : 4 ratio; enantioselectivity is excellent – 97% ee for the cycloaddition product (Scheme 4.19) [28].
It has also been shown by Mikami et al. that a BINOL-titanium(IV) complex in which the 6,6 position of the BINOL ligand is substituted with bromine catalyzes
166 4 Catalytic Enantioselective Cycloaddition Reactions of Carbonyl Compounds
Scheme 4.18
Scheme 4.19
a selective cycloaddition reaction of methyl glyoxylate with 1-methoxy-1,3-diene in up to 81% yield and 97% ee [28c].
A remarkable change in reaction course is notable when changing the metal from aluminum to titanium for cycloaddition reactions using BINOL as the chiral ligand. When the chiral aluminum(III) catalyst is applied the cycloaddition product is the major product, whereas for the chiral titanium(IV) catalyst, the ene product is the major product. The reason for this significant change in reaction course is not fully understood. Maybe the glyoxylate coordinates to the former Le-

4.3 Cycloaddition Reactions of Carbonyl Compounds 167
wis acid complex in a monodentate fashion, whereas in the latter reaction the glyoxylate coordinates in a bidentate fashion, and these two coordination modes promote the two different reaction courses.
Chiral salen-cobalt(III) complexes can also catalyze the reaction of glyoxylates with activated dienes to give the cycloaddition product in moderate yield and ee [29].
Chiral C2-symmetric bisoxazoline-copper(II) complexes [30, 31] were introduced as catalysts for cycloaddition and ene reactions of glyoxylates with dienes [9] leading to intense activity in the use of these catalyst for different cycloaddition reactions.
For the reaction of ethyl glyoxylate 4a with, e.g., 2,3-dimethyl-1,3-butadiene 5a in the presence of Ph-BOX-CuX2 (R)-21a and t-Bu-BOX-CuX2 (S)-21b catalysts (BOX = bisoxazoline) a nearly 1 : 2 ratio of the cycloaddition product 6a relative to the ene product 7b were obtained (Scheme 4.20). The absolute configuration of the product in these cycloaddition reactions catalyzed by the chiral BOX-copper(II) complexes led to the observation that the 4-tert-butyl-BOX ligand and the 4-phe- nyl-BOX ligand in combination with copper(II) salts give opposite asymmetric induction in the product. Using the copper catalysts derived from the R enantiomer of the phenyl-BOX ligand and the S enantiomer of the tert-butyl-BOX ligand in combination with copper(II) afforded the same S enantiomer of the cycloaddition product.
Scheme 4.20
The enantioselective cycloaddition reaction catalyzed by chiral BOX-copper(II) complexes has been used for conjugated cyclic dienes, e.g. 1,3-cyclohexadiene 5c, as shown in Scheme 4.21 [9, 32]. This cycloaddition reaction is dependent on sol-
