
Cycloaddition Reactions in Organic Synthesis
.pdf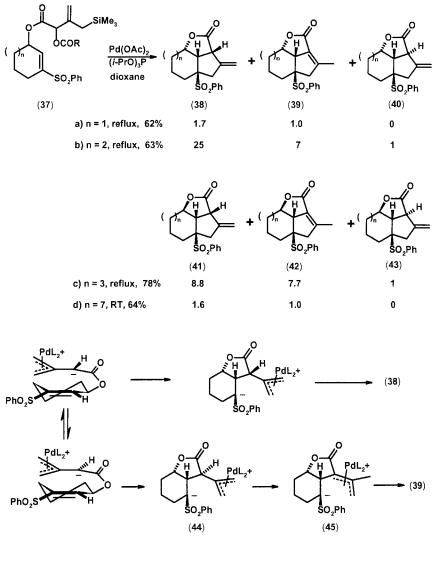
68 2 Recent Advances in Palladium-catalyzed Cycloadditions involving Trimethylenemethane and its Analogs
Scheme 2.13 Intramolecular TMM [3+2] cycloaddition to tricyclic lactones
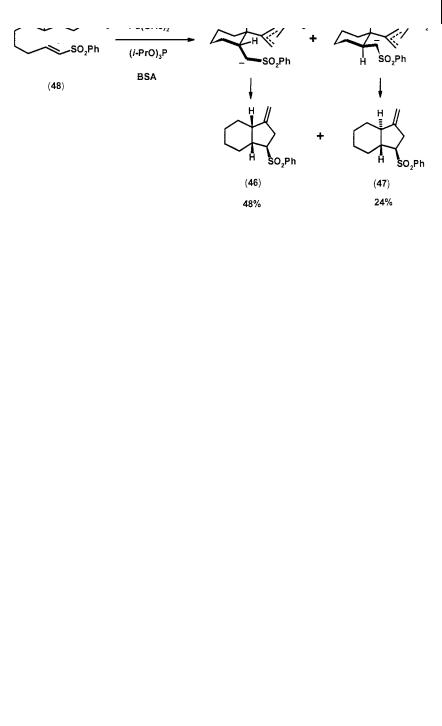
stereospecificity observed in the case of addition to , -unsaturated ester. While the cis olefin geometry of this type of acceptor is normally lost with the parent TMM, it is completely preserved in the cycloadduct (58) derived from the substrate (59) with precursor (56) (Scheme 2.18) [27].
2.4 Application in Organic Synthesis 69
Scheme 2.14 Intramolecular TMM [3+2] cycloaddition to bicyclo[4.3.0]nonyl systems
Scheme 2.15 Acyl effect in intramolecular TMM [3+2] cycloaddition
Scheme 2.16 Intramolecular TMM [3+2] cycloaddition to bicyclo[5.3.0]decanones
70 2 Recent Advances in Palladium-catalyzed Cycloadditions involving Trimethylenemethane and its Analogs
Scheme 2.17 Mechanism of carboxylative TMM cycloaddition
Scheme 2.18 Examples of carboxylative TMM cycloaddition

2.4 Application in Organic Synthesis 71
2.4.6
Carbonyl Cycloadditions
2.4.6.1 Addition to Aldehydes
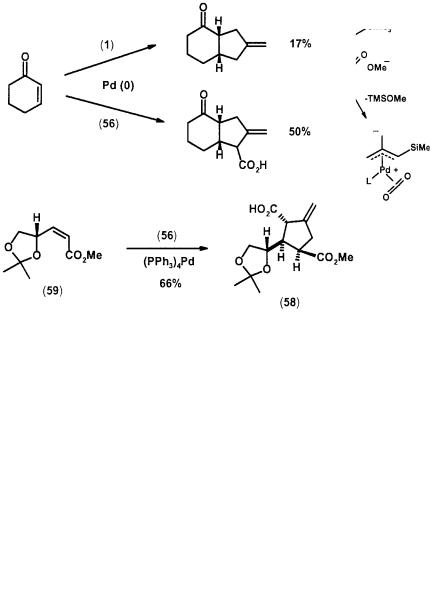
The challenge with adopting the TMM [3+2] strategy in generating a methylenetetrahydrofuran (60) by addition to an aldehyde lies in the poor nucleophilicity of the intermediate alkoxide (61) for intramolecular attack on the -allyl ligand. Therefore, closure to the five-membered ring is often slow compared with the side reaction of silylation by TMSOAc to produce (62), or simply base decomposition (Scheme 2.19). For example, reaction of n-heptanal and cinnamaldehyde with (1) and catalytic (PPh3)4Pd in refluxing THF produced only addition products (62, R = n-C6H13) and (62, R = PhCHCH) in 27% and 45% yield respectively [30]. However, in the presence of a triisopropyl phosphite palladium catalyst and with slow addition of (1) in refluxing dioxane, cinnamaldehyde does produce the desired tetrahydrofuran (60, R = PhCHCH) in 75% yield. A handful of other aldehydes, such as naphthaldehyde and perillaldehyde, also undergo cycloaddition when using this special condition. Nonetheless, these cycloadditions remain capricious as reaction with saturated aldehydes produced only intractable mixture [31].
Scheme 2.19 TMM [3+2] cycloaddition of aldehyde to methylenetetrahydrofuran
On the other hand, the corresponding tin precursor (63) undergoes smooth cycloaddition with a wide variety of aldehydes to produce the desired methylenetetrahydrofuran in good yields [32, 33]. Thus prenylaldehyde reacts with (63) to give cleanly the cycloadduct (64), whereas the reaction with the silyl precursor (1) yields only decomposition products (Scheme 2.20) [31]. This smooth cycloaddition is attributed to the improved reactivity of the stannyl ether (65) towards the -allyl ligand. Although the reactions of (63) with aldehydes are quite robust, the use of a tin reagent as precursor for TMM presents drawbacks such as cost, stability, toxicity, and difficult purification of products.
Remarkably, the addition of only 5–10 mol% of Me3SnOAc to the reactions of the silyl precursor (1) with aldehydes also cleanly produce the cycloadducts in excellent yields [31]. It then appears that the capping of the alkoxide (61) to form the stannyl ether in situ is efficient enough that only a catalytic amount of Me3SnOAc is sufficient to facilitate reaction. This “tin-effect” greatly enhances the

72 2 Recent Advances in Palladium-catalyzed Cycloadditions involving Trimethylenemethane and its Analogs
Scheme 2.20 TMM cycloaddition of tin precursor (63) with prenylaldehyde
generality and efficiency of TMM cycloaddition to carbonyl compounds. Even the enolizable aldehyde (66) produced the tetrahydrofuran adduct (67) in quantitative yield with added Me3SnOAc (Eq. 4). The use of n-Bu3SnOAc gave a somewhat lower yield.
!4"
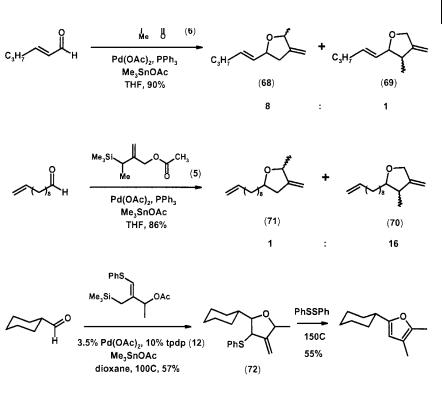
Substituted TMM complexes also cycloadd to aldehydes in the presence of a tin cocatalyst such as Me3SnOAc and Me3SnOTs [31]. Reaction of 2-heptenal with methyl precursor (6) gave a mixture of methylenetetrahydrofurans (68) and (69). This regioselectivity is reversed with 10-undecenal and methyl precursor (5), where adduct (70) now predominates over (71). As in the carbocyclic system, the phenylthio group also functions as a regiocontrol element in reaction with cyclohexyl aldehyde. The initially formed adduct (72) eliminates the element of thiophenol on attempted allyl rearrangement, and the overall process becomes a cycloaddition approach to furans (Scheme 2.21) [20].
2.4.6.2 Addition to Ketones
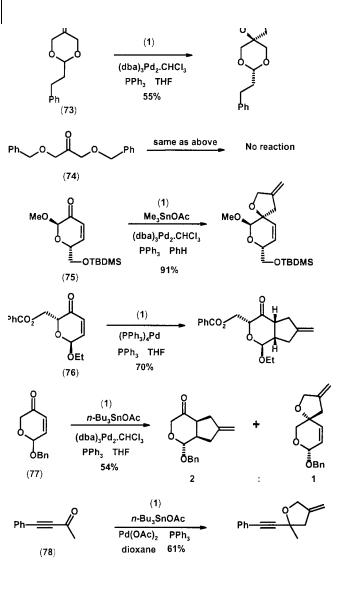
TMM cycloadditions to cyclic and conjugated ketones have also been reported (Scheme 2.22) [31]. The steric nature of the substrate does play a critical role in determining product formation. Thus the cyclic ketone (73) produced 55% yield of the tetrahydrofuran, but no cycloadduct could be obtained from the cyclic ketone (74). The enone (75) gave only carbonyl cycloaddition, whereas enone (76) yielded only olefin adduct. Interestingly, both modes of cycloaddition were observed with the enone (77). The ynone (78) also cycloadds exclusively at the carbonyl function [34].
2.4 Application in Organic Synthesis 73
Scheme 2.21 Tin effect in substituted TMM cycloadditions with aldehydes
2.4.7
Imine Cycloadditions
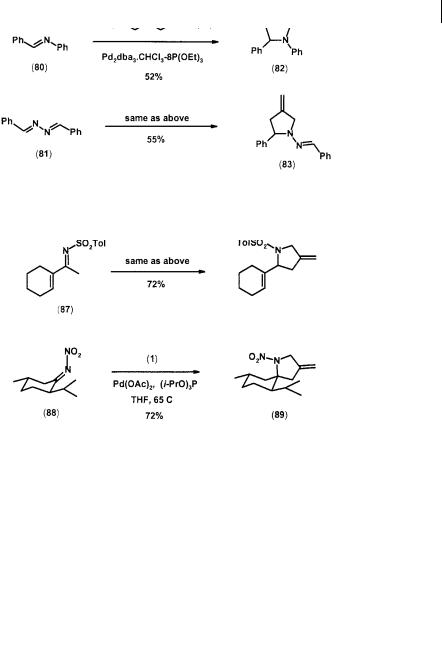
The acetate (1) and its mosylate analog (79) have been shown to undergo cycloaddition with the CN double bond of alkyl imines to generate substituted pyrrolidines in the presence of nickel or palladium catalyst [35]. For example, both the phenyl imine (80) and the diazene (81) gave reasonable yields of adducts (82) and (83) respectively (Scheme 2.23).
Imines with an electron-withdrawing group at the nitrogen atom are excellent acceptors for the acetate (1) or the carbonate (13) [36]. Thus, N-tosylimines (84) gave very good yields of pyrrolidines (85) under typical conditions. The strained cyclic imine (86) and , -unsaturated imine (87) both participated smoothly in the cycloadditions. The hindered nitrimine (88) also reacts well with (1) (but not with 13) to produce the pyrrolidine (89) with a 17 : 1 diastereoselectivity. However, the unhindered nitrimines from cyclohexanone and 2-nonanone failed to react presumably due to enolization (Scheme 2.24).
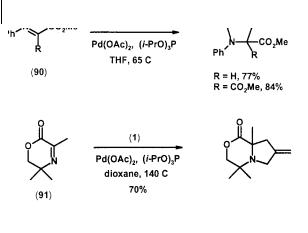
Placing an electron-withdrawing group on the carbon atom of the imine also increases its reactivity toward TMM cycloaddition. Glyoxalate imine (90, R = H) and
74 2 Recent Advances in Palladium-catalyzed Cycloadditions involving Trimethylenemethane and its Analogs
Scheme 2.22 TMM [3+2] cycloadditions with ketones
ketomalonate imine (90, R = CO2Me) both formed cycloadducts in good yields, but the cyclic pyruvate imine (91) still required elevated temperature for reaction (Scheme 2.25) [36].
Substituted TMMs also participate smoothly in imine cycloaddition to generate more structurally elaborate pyrrolidines. The regioselectivity of these reactions is similar to that of olefin addition, although subsequent isomerization of the initial adduct is often observed. For example, the cyano system produced the thermody-
2.4 Application in Organic Synthesis 75
Scheme 2.23 TMM [3+2] cycloadditions with imines
Scheme 2.24 TMM [3+2] cycloadditions with N-activated imines
76 2 Recent Advances in Palladium-catalyzed Cycloadditions involving Trimethylenemethane and its Analogs
Scheme 2.25 TMM [3+2] cycloadditions with C-activated imines
namically more stable endocyclic conjugated isomer (92) [36]. A sulfide shift also accompanied the cycloaddition of imine (93) with the thio-substituted TMM analog to produce (94) as the major product. This allyl sulfide can serve as precursor to a pyrrole synthesis (Scheme 2.26) [20].
2.4.8
[4+3] Cycloadditions
TMM-Pd cycloaddition with electron-deficient 1,3-dienes provides a potentially facile route to seven-membered ring systems if the competing formation of fivemembered ring products can be adequately suppressed. The stepwise mechanism of Michael addition followed by ring-closure is also believed to be operative in this case. Treatment of dimethyl (E,E)-muconate with (1) in the presence of a Pd0 catalyst has been shown to give a mixture of methylenecycloheptene (95) and methylenecyclopentane (96), with the latter being the predominant product under typical conditions (Scheme 2.27). The use of lower reaction temperature and triisopropyl phosphite as the ligand increases the amount of seven-membered ring formation to about even with the five-membered ring product. Interestingly, a phenylsubstituted TMM yielded only the five-membered ring adduct in the reactions with the same muconate and the corresponding (E,Z) isomer [37].
Geometrically restricting the diene to a cisoid conformation greatly enhances the formation of the seven-membered ring. The cycloheptene (97) could be obtained in 65% yield (along with 8% of the spiro-methylenecyclopentane) from the diene (98). Further selective elaboration of (97) demonstrates the versatility of this approach, as the ketone (99) and the dienone (100) can be envisioned as intermediates toward the synthesis of procurcumenol and helispendiolide, respectively. The less sterically hindered diene ester (101) gave exclusively the seven-membered ring adduct as a mixture of diastereoisomers (Scheme 2.27) [38].

2.4 Application in Organic Synthesis 77
Scheme 2.26 Substituted TMM [3+2] cycloadditions with imines
Pyrones also serve as geometrically constrained 4 -electron substrates. The parent and alkyland aryl-substituted pyrones undergo only [4+3] cycloaddition. The bicyclic pyrones (102) give rise to bridged tricyclic adducts in excellent yields. However, ester substitution on pyrone, such as the case in (103), tends to increase the amount of methylenecyclopentane formation [39]. A thio-substituted TMM analog reacts with the pyrone (104) to produce bridged product (105) in good yield (Scheme 2.28) [20]. The pyrone cycloadducts are very useful synthetically. The endocyclic olefin is available for further stereocontrolled elaboration. The lactone bridge represents a cis-1,4-substitution of carbon and oxygen, and a thermal elimination of CO2 can lead to the formation of a cycloheptadiene.
The TMM [4+3] cycloaddition to pyrone has been employed in a synthetic study of a novel biologically active diterpene pseudolaric acid B (106), in which the formation of the bridged adduct (107) from the 2-pyrone (108) is the key step in the sequence (Scheme 2.29). A mixture of the other isomer (109) and the methylenecyclopentane (110) was also isolated from the reaction. It is important to point out that the presence of a tin co-catalyst is critical in effecting the reaction. This is the first example a “tin-effect” observed in a [4+3] cycloaddition [40].
