
Multiple Bonds Between Metal Atoms / 09-Ruthenium Compounds
.pdf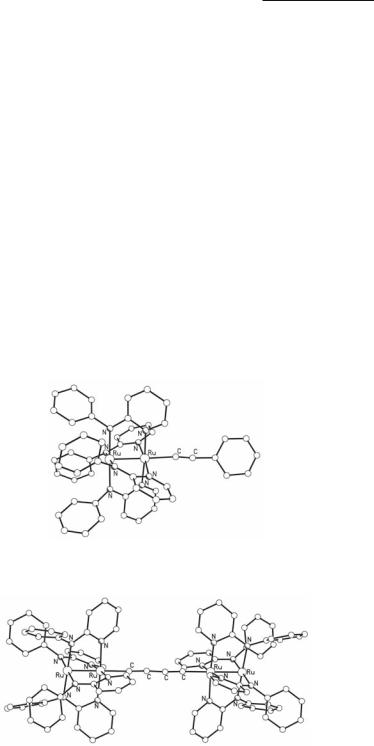
Ruthenium Compounds 397
Angaridis
In most Ru2(Xap)4Cl compounds the polar arrangements (4,0) and (3,1) are preferred even though sometimes they result in distortions of the eclipsed geometry of the paddlewheel structures due to the steric requirements of the aryl substituents of the bridging aminopyridinate ligands, as shown in the structures of the (3,1) and (4,0) regioisomers of Ru2(2,4,6-F3ap)4Cl which display torsion angles of ~17 and ~24º, respectively.109 It has been suggested that this is due to the strong preference of the Ru2 complexes for axial coordination: in the (4,0) regioisomer all the aryl substituents surround one axial site, leaving the other axial site unencumbered allowing the coordination of the Cl- ion. However, there are cases in which other than the (4,0) and (3,1) arrangements are preferred, depending on the basicity of the aminopyridinate ligands. For example, while Ru2(2-Meap)4Cl is obtained only as the (4,0) regioisomer, Ru2(F5ap)4Cl is obtained as a mixture of all possible regioisomers.109,110
The axial Cl- ion of Ru25+ aminopyridinates can be replaced by other ligands upon reactions with suitable reagents. For example, Ru2(ap)4Cl reacts with AgSbF6 in wet MeOH to give [Ru2(ap)4(H2O)](SbF6).111 In addition, Ru2(ap)4Cl reacts with LiC>CPh in 1:5 ratio resulting in the formation of the mono-alkynyl Ru25+ complex Ru2(ap)4(C>CPh) (Fig. 9.14).112 A number of similar complexes of the type Ru2(ap)4[(C>C)mY] (m = 1, 2 and Y = H, SiMe3, CH2COMe) have been obtained by this method.113-115 An attractive extension of such reactions is the synthesis of complexes composed of two Ru25+ tetraaminopyridinate units linked through the axial positions with linear alkynyl-type of ligands, such as [(ap)4Ru2](µ-C>C)[Ru2(ap)4] and [(ap)4Ru2](µ-C>CC>C)[Ru2(ap)4] (Fig. 9.15).116,117 These are synthesized by treating Ru2(ap)4Cl with an excess of the corresponding dilithiated alkynyl reagent.
Fig. 9.14. The structure of Ru2(ap)4(C>CPh).
Fig. 9.15. The structure of [(ap)4Ru2](µ-C>CC>C)[Ru2(ap)4].

398Multiple Bonds Between Metal Atoms Chapter 9
The axial Cl- ions can also be replaced by CN- in stoichiometric reactions giving monocyano adducts,118 or other cyanide-containing mononuclear organometallic complexes resulting in the formation of compounds such as {Ru2(ap)4[NCFe(dppe)(δ5-C5H5)]}+ and {Ru2(ap)4[NCRu(PPh3)2(δ5-C5H5)]}+.119
The Ru25+ tetraaminopyridinates react with strong /-acceptors, like NO, without decomposition. More specifically, Ru2(2-Fap)4Cl reacts with NO to form the axial NO-adduct Ru2(2-Fap)4Cl(NO) in which the bridging ap ligands adopt the (3,1) arrangement in order to minimize the steric repulsions with the phenyl substituents.120 Finally, the complex Ru2(O2CMe)2(ap)2Cl(Hap) reacts with dmpm in the presence of Me3SiCl and NaBPh4 to form the Ru24+ compound [Ru2(ap)2(dmpm)2Cl]BPh4, which is the only crystallographically characterized Ru24+ compound with bridging aminopyridinate ligands.121
Cyclic voltammetry measurements of Ru25+ tetraaminopyridinates show that their electrochemical behavior is strongly influenced by the solvent. Electrochemical measurements for a series of complexes conducted in THF, DMF and DMSO show a single one-electron, metal-centered oxidation and two one-electron, metal-centered reduction processes.122 However, in CH2Cl2 one reduction and two oxidation processes are observed which are assigned to Ru25++ e- Α Ru24+, Ru25+ Α Ru26+ + e- and Ru26+ Α Ru27+ + e-, respectively. These processes are sensitive to the isomer type.109 For example, the potential of the first oxidation for the (3,1) regioisomer of Ru2(F5ap)4Cl in CH2Cl2 is shifted cathodically by ~170 mV compared to that of the (4,0) regioisomer, while the analogous process for the trans-(2,2) regioisomer is shifted cathodically by ~320 mV.110 The potentials of the first oxidation and the reduction processes are also influenced by the substituents on the aryl groups of the aminopyridinate ligands and linear free-energy relationships have been established between the electrode potentials for these processes and the Hammett parameters of the substituents.109
The axial ligands also influence the redox behavior of Ru25+ tetraaminopyridinates significantly. The cyclic voltammogram of Ru2(2-Fap)4Cl(NO) in CH2Cl2 shows two reversible, oneelectron reductions and a reversible, one-electron oxidation at potentials which are shifted to more positive values compared to those in Ru2(2-Fap)4Cl.120 The stabilization of the low-valent redox level of the Ru2 core is explained by the strong /-accepting ability of the NO ligand. Complexes with alkynyl ligands of the type Ru2(ap)4[(C>C)mY] (m = 1, 2, and Y = H, SiMe3, CH2COMe) undergo two one-electron redox processes, a reduction and an oxidation.113 In this case a cathodic shift of the potentials, relative to the analogous processes in the parent complex Ru2(ap)4Cl is observed. This is attributed to the strong nucleophilic character of the C>CR ligands. For complexes of the type [(ap)4Ru2][µ-(C>C)n][Ru2(ap)4] (n = 1, 2, 3, 4, and 6), cyclic voltammetry measurements show that the linear alkynyl chains mediate significant electronic communication between the Ru25+ units. While the mono-alkynyl complex Ru2(ap)4(C>CPh) shows two quasi-reversible redox processes (an oxidation and a reduction), the compound [(ap)4Ru2](µ-C>C)[Ru2(ap)4] exhibits four quasi-reversible, and one irreversible, one-electron redox processes. The strength of the electronic communication decreases as the length of the carbon chain increases.116,117
Complexes with a mixed set of bridging ligands of the type Ru2(O2CMe)4-x(admp)xCl (x = 1, 2, 3) exhibit two redox processes, a one-electron oxidation which becomes easier as the number of aminopyridinate ligands increases, and an one-electron reduction of increasing difficulty with the number of aminopyridinate ligands.108 However, the analogous mixed-ligand Ru25+ complex Ru2(O2CMe)(HNC5H3NMe)3Cl shows three metal-based redox processes, which have been assigned to the oxidation of the Ru25+ core to Ru26+ and the reductions to Ru24+ and further to a rare Ru23+ species.123

Ruthenium Compounds 399
Angaridis
As shown in Table 9.1, the Ru–Ru bond lengths in Ru25+ aminopyridinates fall in the range of 2.274 to 2.336 Å (Ru2(2-Fap)4Cl(NO) is an exception; see below). Although these distances do not show any dependence on the arrangement and the substitution of the bridging aminopyridinate ligands, they are significantly affected by the axial coordination: the complexes with axial alkynyl ligands exhibit longer Ru–Ru bond lengths. For example, the Ru–Ru bond distances in Ru2(ap)4(C>CPh)112 and Ru2(ap)4(C>CC>CSiMe3)114 are 2.319(3) and 2.330(1) Å, respectively, and they are longer than that of 2.275(3) Å in the parent complex Ru2(ap)4Cl. These rather elongated Ru–Ru bonds are attributed to the electron donating character of the alkynyl ligands, which result in an increase of the anti-bonding μ* electron density between the two metal atoms and weakening of the Ru–Ru bond.
The compound Ru2(2-Fap)4Cl(NO) exhibits a Ru–Ru bond length of 2.420(1) Å, which is significantly longer than that in all other Ru25+ tetraaminopyridinate complexes.120 Given the nature of NO, this could reflect a reduction of the Ru25+ core to Ru24+. Indeed, the formulation Ru24+(NO)+ is supported by an almost linear Ru–N–O angle. The lowering of the oxidation state from Ru25+ to Ru24+ implies addition of an electron to the anti-bonding orbitals of the dimetal unit which results in the observed lengthening of the Ru–Ru bond.
Room temperature magnetic measurements for the Ru25+ tetraaminopyridinates show magnetic moments in the range 3.8-4.0 BM, indicating the presence of three unpaired electrons.110,112,124 Considering that the Ru–Ru bond lengths of Ru25+ tetraaminopyridinates fall almost in the same range as those reported for Ru25+ tetracarboxylates, the two types of compounds should exhibit the same electronic configuration, i.e., μ2/4β2(/*β*)3. Room temperature magnetic measurements conducted for the series of complexes with a mixed set of bridging admp/acetate ligands of the type Ru2(O2CMe)4-x(admp)xCl (x = 1, 2, 3) also indicate the presence of three unpaired electrons. However, the magnetic moment of Ru2(admp)4Cl implies the presence of only one unpaired electron.108 The explanation that was given is that the four admp ligands cause a destabilization of the β* orbital resulting in the μ2/4β2/*3 electronic configuration.
Formamidinate ligands
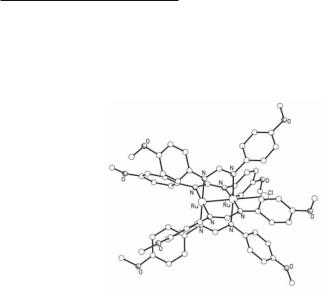
Ru25+ tetraformamidinates are synthesized from the reactions of Ru2(O2CMe)4Cl with excess of molten formamidines (HDArF), a method that was used for the synthesis of the first complex of this type to be reported, Ru2(DTolF)4Cl.125 Alternatively, they can be synthesized from stoichiometric ligand metathesis reactions by refluxing Ru2(O2CMe)4Cl with the appropriate formamidine in the presence of Et3N in THF.126 By careful control of the reaction conditions or by using formamidines with appropriate substituents on the aryl rings complexes with a mixed set of formamidinate/acetate ligands of the type Ru2(O2CMe)4-x(DArF)xCl (x = 1, 2, 3) can be synthesized in a controlled manner.127-130 For example, the reaction of Ru2(O2CMe)4Cl with HDAniF in 1:2 ratio in refluxing THF (~70ºC) results in the synthesis of cis-Ru2(O2CMe)2(DAniF)2Cl.128 In contrast, the reaction of Ru2(O2CMe)4Cl with HDXyl2,6F gives the bis-substituted complex Ru2(O2CMe)2(DXyl2,6F)2Cl only at ~150 ºC, while the DXyl2,6F ligands are forced in a transoid arrangement due to the steric requirements imposed by the methyl substituents of the aryl rings.131 In addition, the reaction of Ru2(O2CMe)4Cl with excess of HDAniF in refluxing toluene gives the fully substituted complex Ru2(DAniF)4Cl, while the analogous reaction in boiling MeOH is not a substitution reaction but a disproportionation, which results in the Ru24+ complex Ru2(DAniF)4 and the edge-sharing bioctahedral Ru(III)Ru(III) compound [Ru2(OMe)2(O2CMe)2(HDAniF)4]Cl2.131
In the solid state, Ru25+ tetraformamidinates exist as discrete paddlewheel structures, which do not associate (either via the axial Cl- ions as in Ru25+ carboxylate compounds, or directly as
400Multiple Bonds Between Metal Atoms Chapter 9
in Ru25+ oxopyridinates and aminopyridinates) due to the steric requirements of the aryl groups of the bridging formamidinate ligands. An example of a Ru25+ tetraformamidinate complex is shown in Fig. 9.16.131 Ligands known to coordinate axially are Cl- ions, alkynyl groups, and solvent molecules, such as THF.
Fig. 9.16. The structure of Ru2(DAniF)4Cl.
The axial Cl- ion of Ru2(DArF)4Cl complexes can be replaced either by strongly coordinating solvent molecules (e.g., MeOH), or by anionic ligands, such as alkynyls. For example, reaction of Ru2(DPhF)4Cl with LiC>CPh in 1:5 ratio gives Ru2(DPhF)4(C>CPh).132 A few other similar compounds of the type Ru2(DArF)4[(C>C)mY] (Ar = Ph, Phm-Cl, Ph3,5-diCl, Anim, Y = Ph, SiMe3, m = 1, 2) have been synthesized by this method.115,133
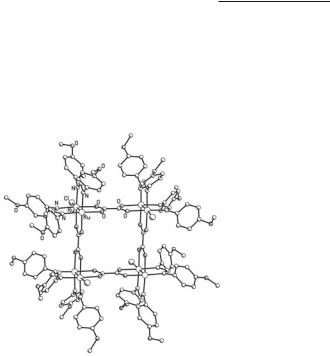
In complexes with a mixed set of formamidinate and acetate bridging ligands substitution of the labile acetate ligands can take place. The reaction of Ru2(O2CMe)(DPhF)3Cl with p-(n-decyloxy)benzoic acid results in Ru2(O2CC6H4-p-OC10H21)(DPhF)3Cl,130 whereas the reaction of Ru2(O2CMe)(DAniF)3Cl with the dicarboxylic acid 1,4-HO2CC6H4CO2H gives the molecular pair [Ru2(DAniF)3Cl](µ-O2CC6H4CO2)[Ru2(DAniF)3Cl].129 In an analogous way, cis-Ru2(O2CMe)2(DAniF)2Cl reacts with the dicarboxylic acids HO2CCO2H and HO2CC6H4CO2H to form the molecular squares {[cis-Ru2(DAniF)2Cl](µ-O2CCO2)}4 (Fig. 9.17) and {[cis-Ru2(DAniF)2Cl](µ-O2CC6H4CO2)}4, respectively.128
Cyclic voltammetry measurements of Ru2(DArF)4Cl complexes show a reversible, one electron, metal-based oxidation process and an irreversible reduction process, which correspond to Ru25+ Α Ru26+ + e- and Ru25+ + e- Α Ru24+, respectively.125,126,134 In some cases an additional irreversible redox wave has also been observed, which was assigned to the axial chloride-free redox couple Ru25+/Ru24+. The potentials of these processes are dependent on the substitution on the aryl groups of the ligand and linear correlations between the electrode potentials of the redox processes and the substituent’s Hammett constants have been established.126 The monoalkynyl Ru25+ tetraformamidinate complexes exhibit analogous redox processes, an irreversible oxidation and a reversible reduction, but the electrode potentials of the redox waves are cathodically shifted compared to those of the corresponding Ru2(DArF)4Cl compounds.133
The Ru–Ru bond distances for Ru25+ formamidinates lie in a wide range of 2.305 to 2.506 Å (Table 9.1). These distances, which are longer than those observed in the Ru25+ tetracarboxylates, do not depend on the substituents of the aryl groups, but they are strongly influenced by the nature of the axial ligand. Shorter Ru–Ru bond lengths are observed in complexes in which the axial ligand is a Cl- ion, while longer ones are observed when the axial ligand is an alkynyl anion, e.g., the Ru–Ru distance in Ru2(DAnimF)4(C>CC>CSiMe3) is 2.506(1) Å.135 The alkynyl
Ruthenium Compounds 401
Angaridis
bonding interaction with the Ru25+ core has been studied in a series of Ru2(DArF)4(C>CPh) complexes using IR spectroscopy.133 Based on the dependence of ι(C>C) on the substituents of the formamidinates, it was concluded that there is a strong Ru–C_ μ-bonding interaction (d/–/ back bonding interaction is also present in a small degree). This strong μ-bonding interaction increases the antibonding μ* electron density between the two metals resulting in the lengthening of the Ru–Ru bond.
Fig. 9.17. The structure of the molecular square {[cis-Ru2(DAniF)2Cl](µ-O2CCO2)}4.
The Ru25+ tetraformamidinates exhibit room temperature magnetic moments in the range 3.64-3.97 BM, which is indicative of the presence of three unpaired electrons and corresponds to the μ2/4β2(/*β*)3 electronic configuration.126 In addition, the temperature dependence of the magnetic moment of Ru2(DTolF)4Cl at 300 K shows that its magnetic moment has a value of 3.66 BM, but at temperatures below ~100 K there is a deviation from the Curie-Weiss behavior, as the magnetic moment decreases. This deviation was ascribed to zero-field splitting (D ~50 cm-1), since any type of interdimer antiferromagnetic interaction was excluded.125
Room temperature magnetic susceptibility measurements for complexes with a mixed set of bridging formamidinate/acetate ligands have also corresponded to the μ2/4β2(/*β*)3 electronic configuration.127-129 In the case of the molecular squares {[cis-Ru2(DAniF)2Cl](µ-O2CCO2)}4 and {[cis-Ru2(DAniF)2Cl](µ-O2CC6H4CO2)}4 variable temperature magnetic susceptibility studies show that in the square with the shorter oxalate bridges there is a weak antiferromagnetic coupling between the Ru25+ units (ε ~ -5 K), while in the terephthalate analog the coupling is negligible.128 Analogously, no coupling was observed between the two Ru25+ units in the compound [Ru2(DAniF)3Cl](µ-O2CC6H4CO2)[Ru2(DAniF)3Cl].129
Naphthyridine ligands
There are only two known Ru25+ naphthyridine complexes, Ru2(O2CMe)3(bcnp)136 (Fig. 9.18) and trans-Ru2(O2CMe)2(mephonp)2Cl137 (Fig. 9.19). These were synthesized from the reactions of Ru2(O2CMe)4Cl with the corresponding naphthyridine in MeOH under mild conditions. Complexes with four bridging naphthyridine ligands have not been reported. This is probably due to the fact that the high temperatures and long reaction times that appear to be necessary for the syntheses of the fully substituted complexes are associated with reduction of the Ru25+ core to Ru24+ (see section 9.3.3).

402Multiple Bonds Between Metal Atoms Chapter 9
Fig. 9.18. The structure of Ru2(O2CMe)3(bcnp).
Fig. 9.19. The structure of trans-Ru2(O2CMe)2(mephonp)2Cl.
The crystal structure of trans-Ru2(O2CMe)2(mephonp)2Cl (Fig. 9.19) shows a polar arrangement of the mephonp ligands.137 As for the similar Ru25+ complex trans-Ru2(O2CMe)2- (mhp)2Cl,102 the strong preference of the Ru25+ unit for axial coordination favors the polar and transoid arrangement of the bridging mephonp ligands, regardless of the steric crowding that this preference may cause (there is a twist angle of ~6º). Interestingly, the mephonp ligands, which can adopt either the N,O or the N,N' coordination mode, prefer the N,O coordination, since this leaves one axial site unencumbered for coordination of the Cl- ion that stabilizes the molecule. The Ru–Ru bond lengths in Ru2(O2CMe)3(bcnp) and trans-Ru2(O2CMe)2(mephonp)2Cl are 2.285(1) and 2.265[5] Å, respectively (Table 9.1). These are within the range of the Ru–Ru distances observed in Ru25+ tetracarboxylates. Although there are no magnetic measurements that would give some information about the electronic structure of these complexes, the observed Ru–Ru bond lengths give an indication for the μ2/4β2(/*β*)3 electronic configuration.
Other N,N'-donor bridging ligands
Other N,N'-donor bridging ligands that have been used in complexes with the Ru25+ core include: admpym, a series of 5-Rsalpy ligands, dmat and DTolTA.
Reaction of Ru2(O2CMe)4Cl with Hadmpym in MeOH results in the synthesis of Ru2(O2CMe)3(admpym)Cl(MeOH) which exhibits a Ru–Ru bond length of 2.290(1) Å
Ruthenium Compounds 403
Angaridis
(Table 9.1).138 This complex undergoes three one-electron redox processes, one oxidation and two reductions, which correspond to the processes Ru25+ Α Ru26+ + e-, Ru25+ + e- Α Ru24+, and Ru24+ + e- Α Ru23+, respectively. Variable temperature magnetic susceptibility measurements show a ground state with S = 3/2, arising from the μ2/4β2(/*β*)3 electronic configuration.
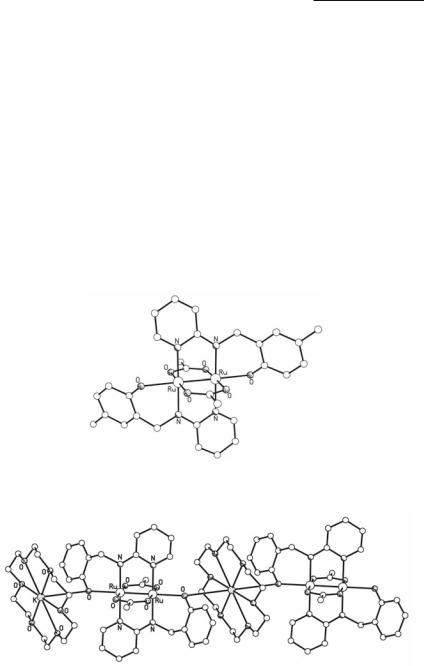
Complexes of the type [Ru2(O2CMe)2(5-Rsalpy)2]- have been synthesized from the reactions of Ru2(O2CMe)4Cl with the dianionic, tridentate ligands 5-Rsalpy (R = H, Me, Cl, Br, NO2) in 1:2 ratio.139,140 In the solid state, these complexes are isolated by using K+ or Na+(18-crown-6) as counter-cations, and they display either discrete paddlewheel structures (Fig. 9.20), or onedimensional polymeric chain structures formed by the interactions of the alkali metals with the phenolate O atoms of the [Ru2(O2CMe)2(5-Rsalpy)2Cl]- units (Fig. 9.21). The 5-Rsalpy ligands are at transoid positions exhibiting a bridging/axial chelating coordination mode. The Ru–Ru bond lengths are in the range 2.283-2.300 Å. Electrochemical studies of these complexes reveal four redox processes: a metal-centered reduction of the Ru25+ core to Ru24+, two metal-centered oxidations to Ru26+ and an unusual Ru27+ core, while a fourth redox process is assigned to a ligand-based oxidation. The temperature dependence of the magnetic susceptibility supports the μ2/4β2(/*β*)3 electronic configuration.
Fig. 9.20. The structure of [Ru2(O2CMe)2(5-Mesalpy)2]-.
Fig. 9.21. The polymeric structure of [K(18-crown-6)][Ru2(O2CMe)2(salpy)2].
A similar reaction of Ru2(O2CMe)4Cl with 5-Clsalpy in 1:3 ratio results in the complete substitution of the acetate ligands and the formation of Li2(THF)4Cl[Ru2(5-Clsalpy)3] with a Ru–Ru bond length of 2.313(1) Å.141 In this complex one of the 5-Clsalpy ligands embraces the dimetal unit in a bridging/axial chelating coordination mode, while the other two ligands adopt a bridging/equatorial chelating coordination mode.

404Multiple Bonds Between Metal Atoms Chapter 9
The complexes Ru2(dmat)4Cl, synthesized from the reaction of Ru2(O2CMe)4Cl with excess of molten Hdmat, and [Ru2(DTolTA)4(MeCN)]BF4, obtained from the reaction of Ru2(DTolTA)4 with AgBF4 in MeCN, exhibit an electronic structure that is not common for Ru25+ complexes.142,169 Their room temperature magnetic moments of 1.70 and 1.88 BM, respectively, are consistent with the presence of one unpaired electron, suggesting either the μ2/4β2β*2/*1 or the μ2/4β2/*3 electronic configuration. The unusually long Ru–Ru distances of 2.432(1) Å of the former and 2.373(1) Å of the latter (compared to other Ru25+ complexes) support the latter electronic configuration, since the presence of three electrons in the /* molecular orbitals is expected to result in a substantial lengthening of the Ru–Ru bond, whereas the lengthening of the Ru–Ru bond caused by the presence of electrons in the β* molecular orbital would have been small. The destabilization of the β* molecular orbital is attributed to an interaction with suitable molecular orbitals of the highly basic dmat and DTolTA ligands.
9.3Ru24+ Compounds
Complexes of the paddlewheel framework with a Ru24+ core, together with those with a Ru26+ core discussed in the following section, represent the other two main families of Ru2 compounds. The Ru24+ compounds that have been structurally characterized along with their corresponding Ru–Ru bond lengths are listed in Table 9.2.
Table 9.2. Structurally characterized Ru24+ paddlewheel compounds
Compound |
r(Ru–Ru) (Å) |
ref. |
O,O'-donor bridging ligands |
|
|
carboxylate ligands |
|
|
Ru2(O2CPh)4(PhCO2H)2 |
2.263(1) |
15 |
[RuCl(MeCN)3(PPh3)2]2[Ru2(O2CC6H4-p-Me)4Cl2]·4H2O |
2.291(2) |
30 |
Ru2(O2CCF3)4(THF)2 |
2.276(3) |
80 |
Ru2(O2CEt)4(NO)2 |
2.515(4) |
80 |
Ru2(O2CCF3)4(NO)2 |
2.532(4) |
80 |
Ru2(O2CMe)4(THF)2 |
2.260(2) |
143 |
Ru2(O2CMe)4(THF)2 |
2.261(3) |
144 |
Ru2(O2CMe)4(H2O)2 |
2.262(3) |
144 |
Ru2(O2CEt)4(acetone)2 |
2.260(3) |
144 |
Ru2(O2CC10H15)4(MeOH)2·2MeOH |
2.281(1) |
147 |
Ru2(O2CCH(OH)Ph)4(H2O)2 |
2.266[2] |
148 |
Ru2(O2CCPh3)4(H2O)(EtOH)·2EtOH |
2.252(2) |
149 |
Ru2(O2CC(O)Ph)4(THF)2 |
2.274(1) |
151 |
Ru2(O2CC6H4-p-Me)4(THF)2·2THF |
2.269(1) |
152 |
Ru2(O2CC6H4-p-Me)4(MeCN)2·3MeCN |
2.276(1) |
152 |
Ru2(O2CCF3)4(phz) |
2.311(1) |
157 |
[Ru2(O2CCF3)4]2(µ4-TCNQ)·3toluene |
2.287(1) |
159 |
Ru2(O2CCF3)4(tempo)2 |
2.293(1) |
163 |
N,O-donor bridging ligands |
|
|
oxopyridinate ligands |
|
|
(4,0)-Ru2(chp)4(THF)·THF |
2.261(1) |
98 |
trans-(2,2)-Ru2(chp)4 |
2.248(1) |
98 |
trans-(2,2)-Ru2(mhp)4·CH2Cl2 |
2.238(1) |
164 |
trans-(2,2)-Ru2(mhp)4 |
2.235(1) |
165 |
trans-(2,2)-Ru2(bhp)4·1.5benzene |
2.259(1) |
165 |

Ruthenium Compounds 405
Angaridis
Compound |
r(Ru–Ru) (Å) |
ref. |
|
[(3,1)-Ru2(chp)4]2·CH2Cl2 |
2.247[1] |
165 |
|
(4,0)-Ru2(fhp)4(THF) |
2.274(1) |
166 |
|
other N,O-donor bridging ligands |
|
|
|
[Ru2(O2CMe)2-x(O2CCF3)x(9-EtGH)2(MeOH)2](O2CCF3)2·2MeOH·0.5Et2O |
2.322(13) |
214 |
|
(x = 0.18) |
|||
|
|
||
N,N'-donor bridging ligands |
|
|
|
formamidinate ligands |
|
|
|
[Ru2(DAniF)3Cl0.12]2(µ-O2CC6H4CO2)·6THF |
2.416(1) |
129 |
|
Ru2(DAniF)4 |
2.454(1) |
131 |
|
Ru2(DTolF)4·2benzene |
2.474(1) |
167 |
|
Ru2(DPhF)4(CO)·4CH2Cl2 |
2.554(1) |
168 |
|
triazenate ligands |
|
|
|
Ru2(DPhTA)4 |
2.399(1) |
169 |
|
Ru2(DTolTA)4(MeCN) |
2.407(1) |
169 |
|
Ru2(DTolTA)4·3toluene |
2.417(2) |
170 |
|
naphthyridine ligands |
|
|
|
trans-Ru2(O2CMe)2(mephonp)2·2CHCl3 |
2.268(2) |
137 |
|
Ru2(mephonp)4(H2O)·0.5MeOH·1.5C6H4Cl2 |
2.238(2) |
137 |
|
cis-[Ru2(O2CMe)2(pynp)2](PF6)2·2MeOH |
2.298(1) |
172 |
|
[Ru2(O2CMe)3(bpnp)]PF6 |
2.28(2) |
173 |
|
Ru2(meonp)4·2benzene |
2.258(2) |
174 |
9.3.1 Ru24+ compounds with O,O'-donor bridging ligands
Carboxylate ligands
Despite the fact that the mild reduction potentials for the Ru25+ tetracarboxylates indicated that the one-electron reduced Ru24+ analogs were chemically accessible,3,48 it was not until 1984 that Wilkinson and coworkers reported the synthesis of the first Ru24+ tetracarboxylate. Ru2(O2CMe)4(THF)2 (Fig. 9.22) was made by reacting Ru2(O2CMe)4Cl with the Grignard reagent Me3SiCH2MgCl (the latter acting as one-electron reducing agent).143 A more efficient synthetic method for Ru24+ tetracarboxylates was reported the following year which involved reactions of Na+ or Li+ salts of the appropriate carboxylic acids with a “blue solution of RuCl3” (a MeOH solution of RuCl3·xH2O that has been reduced with H2). A number of Ru2(O2CR)4L2 complexes (R = H, Me, CH2Cl, Et, Ph, and L = H2O, THF, MeOH, acetone, MeCN) were made following this synthetic method.144 Exchange reactions of Ru2(O2CH)4 (the compound obtained in the best yield from the above mentioned synthetic method) with suitable salts of different carboxylates also result in new Ru24+ tetracarboxylates. However, this procedure fails to give Ru2(O2CCF3)4, whereas the reaction of Ru2(O2CMe)4 with AgO2CCF3 results in Ru2(O2CMe)4(O2CCF3). Ru2(O2CCF3)4 can be made by refluxing Ru2(O2CMe)4 in a CF3CO2H/(CF3CO)2O mixture in the presence of NaO2CCF3,80 or by reaction of RuCl3·xH2O with AgO2CCF3.145
Reduction of Ru25+ tetracarboxylates can also be used for the synthesis of compounds of this type. For example, the complex Ru2(asp)4Cl is converted to Ru2(asp)4(NO) by heating its MeOH solution in the presence of AgNO3.146 In another case K3[Ru2(O2CO)4] reacts with 1-adamantylcarboxylic acid in MeOH/H2O solution to yield the Ru24+ tetraadamantylcarboxylate complex.147 Furthermore, reaction of Ru2(O2CMe)4Cl with mandelic acid gives Ru2(mandelate)4,148 which serves as a starting material in carboxylate exchange reactions to
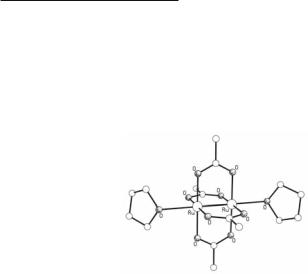
406Multiple Bonds Between Metal Atoms Chapter 9
give other Ru24+ tetracarboxylate compounds.149 Although the mechanism of these reductions is not clear, it has been proposed that they proceed via a disproportionation pathway, which in the case of Ru2(mandelate)4 can be described with the equation:
6Ru2(mandelate)4Cl + 8H2O Α 3Ru2(mandelate)4 + 2[Ru3(µ3-O)(mandelate)6(H2O)3]Cl + 4HCl
Fig. 9.22. The structure of Ru2(O2CMe)4(THF)2.
Alternatively, chemical reduction of Ru25+ tetracarboxylates using either CrCl2,150 or Zn/Hg151 can also be used to prepare their Ru24+ analogs.
The Ru24+ tetracarboxylates have a high affinity for axial coordination. In coordinating solvents they exist as discrete diadducts of the general type Ru2(O2CR)4L2, where L is a solvent molecule. The same type of structure is maintained in the solid state. However, in non-coor- dinating solvents oligomerization takes place by intermolecular interactions between the O atoms of the bridging carboxylate ligand of one molecule with the axial position of another molecule.152
The Ru–Ru bond lengths of the Ru2(O2CR)4L2 compounds fall in the range of 2.252-2.311 Å, (there are two exceptions that are discussed in the following paragraph). They do not show any significant dependence on the substituent R of the carboxylate bridge or the type of the axial donor ligand L. However, they are slightly longer than those in the Ru25+ teracarboxylate analogs (e.g., Ru–Ru = 2.262(3) Å in Ru2(O2CMe)4(H2O)2144 and Ru–Ru = 2.248(1) Å in [Ru2(O2CMe)4(H2O)2]BF4).7
The compounds with metal-metal bond lengths outside of the aforementioned range are Ru2(O2CEt)4(NO)2 and Ru2(O2CCF3)4(NO)2, with Ru–Ru distances of 2.515(4) and 2.532(4) Å, respectively.80 Early attempts to explain these exceptions suggested that the complexes could be considered as RuI–RuI complexes, with both NO ligands donating their odd /* electrons to the Ru2 unit, resulting in the electronic configuration μ2/4β2/*4β*2 and a diamagnetic ground state. However, this is not consistent with the short Ru–N bond lengths, the bent coordination mode of the NO groups ( Ru–N–O = 153º) and the low NO stretching frequencies (17451805 cm-1). Thus, the long Ru–Ru distances are probably due to strong μ interactions, while the rest of the data suggest a strong delocalization of the /* electron density over the ONRuRuNO chain. Theoretical calculations for the Ru2(O2CR)4(NO)2 (R = H, CF3) compounds have been conducted and their PES spectra have been reported.153-155 However, the results of these studies do not lead to a good understanding of the electronic structures of these compounds.
The Ru24+ tetracarboxylates react with Lewis bases (e.g., THF, acetone, H2O) which occupy the axial positions forming Ru2(O2CR)4L2 diadducts.80 These axial ligands can be removed by heating under vacuum to give unsolvated compounds. As for the Ru25+ analogs, their reactions with Lewis bases that are also /-acceptors result in the cleavage of the metal-metal bond.
