
Multidimensional Chromatography
.pdf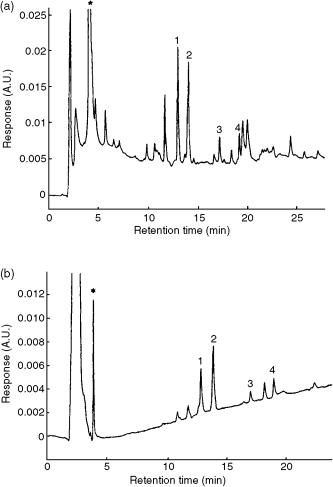
Multidimensional Chromatography in Environmental Analysis |
359 |
Figure 13.14 LC-diode-array detection (DAD) chromatogram (at 220 nm) obtained after preconcentration of 50 ml of ground water sample spiked with various pollutants at levels of 3 g l 1 passed through (a) a PLRP-S cartridge and (b) an anti-isoproturon cartridge. Peak identification is as follows: 1, chlortoluron; 2, isoproturon plus diuron; 3, linuron; 4, dibenzuron; *, water matrix. Reprinted from Journal of Chromatography, A 777, I. Ferrer et al. ‘Automated sample preparation with extraction columns by means of anti-isoproturon immunosorbents for the determination of phenylurea herbicides in water followed by liquid chromatography diode array detection and liquid chromatography – atmospheric pressure chemical ionization mass spectrometry’, pp. 91 – 98, copyright 1997, with permission from Elsevier Science.
if a retention gap is used. The techniques developed for this can also be used to transfer relevant fractions from an LC – column and this has been extremely important in developing LC – GC methods for environmental trace analysis.
360 |
Multidimensional Chromatography |
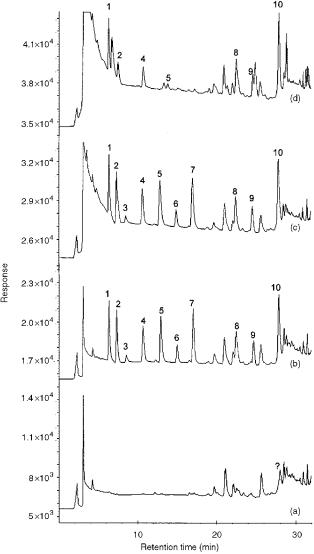
Figure 13.15 Chromatograms obtained by on-line trace enrichment of 50 ml of Ebro river water with and without the addition of different volumes of 10% Na2SO3 solution for every 100 ml of sample: (a) blank with the addition of 1000 l of sulfite; (b) spiked with 4 g l 1 of the analytes and 1000 l of sulfite; (c) spiked with 4 g l 1 of the analytes and 500 l of sulfite; (d) spiked with 4 g l 1 of the analytes without sulfite. Peak identification is as follows: 1, oxamyl; 2, methomyl; 3, phenol; 4, 4-nitrophenol; 5, 2,4-dinitrophenol; 6, 2-chlorophenol; 7, bentazone; 8, simazine; 9, MCPA; 10, atrazine. Reprinted from Journal of Chromatography, A 803, N. Masqué et al., ‘New chemically modified polymeric resin for solid-phase extraction of pesticides and phenolic compounds from water’, pp. 147 – 155, copyright 1998, with permission from Elsevier Science.
Multidimensional Chromatography in Environmental Analysis |
361 |
LC – GC is a very powerful analytical technique because of its selectivity and sensitivity in analysing complex mixtures and therefore it has been used extensively to determine trace components in environmental samples (2, 5, 77). LC allows preseparation and concentration of the components into compound types, with GC being used to analyse the fractions. The advantages of on-line LC– GC over the off-line system are, first, the less sample which is required and, secondly, that there is less need for laborious sample pretreatment because the method is automated (78).
Important developments in LC– GC have been made by Grob and co-workers (79 – 81) and by the Brinkman group (82 – 87), who have mainly studied the application of this technique to environmental analysis. This coupled technique has usually been applied to water, although air and soil extracts have also been analysed.
On-line coupling of normal-phase liquid chromatography (NPLC) and gas chromatography is today a well developed and robust procedure and has been regularly applied to environmental analysis. When a fraction of the NPLC sample is introduced in to the GC unit, a large-volume interface (LVI) is needed but, due to the volatility of the organic solvent used in NPLC, this does not present such a great problem.
However, for water analysis, reverse-phase liquid chromatography is more suitable but its coupling with GC has some drawbacks because of the partly aqueous effluent. Several systems have been developed (88, 89) and applied to determine pollutants in water.
An alternative way of eliminating water is to pass the aqueous sample through a small LC column, also called a precolumn or an SPE column, where the analytes are retained and the water is mostly eliminated. Subsequent elution of these analytes with an organic solvent (usually ethyl or methyl acetate) and more compatible handling with GC systems enables the sample to be significantly concentrated and low levels of analytes to be determined. However, an additional step is needed prior to the elution of the retained analytes in order to eliminate the small amounts of water in the LC column. This is usually carried out by drying the LC column with nitrogen (89) or by adding a small drying column containing sodium sulfate or silica (89, 90). The former approach is normally used and we can find several applications to environmental analysis in the literature (91 – 93).
These small columns,(usually 10 mm 1 – 4.6 mm i.d.) are normally packed with 10 – 40 m sorbents such as C18-bonded silica, C8-bonded silica or styrene – divinylbenzene copolymer. These sorbents are not very selective and more selective sorbents, such as the immunosorbent (94), have also been used with good results. Coupling of SPE – gas chromatography is in fact the one most often used in environmental analysis because it reaches a high level of trace enrichment, eliminates water and elutes retained compounds easily with an organic solvent that can be injected into the gas chromatograph.
In order to achieve the widest application range, partially concurrent solvent evaporation (PCSE) with an on-column interface is normally used during the transfer of analytes from the LC-type precolumn to the GC system. Fully concurrent solvent evaporation (FSCE), with a loop-type interface, is used in some cases, although the
362 |
Multidimensional Chromatography |
more volatile analytes can be lost. A co-solvent (an organic solvent with a higher boiling point than the transfer solvent) can be added before the transfer step to minimize this effect (95).
A programmed-temperature vaporizer (PTV) has also been used as an interface for introducing the LC fraction to the GC unit (84,96) and to desorb the analytes retained in the SPE sorbent contained in the PTV liner. Water samples can then be injected directly in to the PTV injector.
13.4.2 EXAMPLES OF LIQUID CHROMATOGRAPHY – GAS CHROMATOGRAPHY APPLIED TO ENVIRONMENTAL ANALYSIS
One example of normal-phase liquid chromatography coupled to gas chromatography is the determination of alkylated, oxygenated and nitrated polycyclic aromatic compounds (PACs) in urban air particulate extracts (97). Since such extracts are very complex, LC – GC is the best possible separation technique. A quartz microfibre filter retains the particulate material and supercritical fluid extraction (SFE) with CO2 and a toluene modifier extracts the organic components from the dust particles. The final extract is then dissolved in n-hexane and analysed by NPLC. The transfer at 100l min 1 of different fractions to the GC system by an on-column interface enabled many PACs to be detected by an ion-trap detector. A flame ionization detector (FID) and a 350 l loop interface was used to quantify the identified compounds. The experimental conditions employed are shown in Table 13.2.
Figure 13.16 shows the LC separation of this extract and the GC/FID chromatogram of the oxy-PAC fraction. The different oxy-PACs can be quantified without interfering compounds with a non-selective detector such as an FID.
One of the first examples of the application of reverse-phase liquid chromatography – gas chromatography for this type of analysis was applied to atrazine (98). This method used a loop-type interface. The mobile phase was the most important parameter because retention in the LC column must be sufficient (there must be a high percentage of water), although a low percentage of water is only possible when the loop-type interface is used to transfer the LC fraction. The authors solved this problem by using methanol/water (60:40) with 5% 1-propanol and a precolumn. The experimental conditions employed are shown in Table 13.2.
An alternative way of eliminating water in the RPLC eluent is to introduce an SPE trapping column after the LC column (88, 99). After a post-column addition of water (to prevent breakthrough of the less retained compounds), the fraction that elutes from the RPLC column is trapped on to a short-column which is usually packed with polymeric sorbent. This system can use mobile phases containing salts, buffers or ion-pair reagents which can not be introduced directly into the GC unit. This system has been successfully applied, for example, to the analysis of polycyclic aromatic hydrocarbons (PAHs) in water samples (99).
Another interface for RPLC – GC is the programmed-temperature-vaporization (PTV) system, an interesting application of which is the determination of phthalates

Table 13.2 Examples of the application LC – GC in environmental analysis
|
|
Sample |
|
Interface/ |
|
|
LOD b |
|
|
|
|
volume |
LC column |
Transfer |
GC system |
|
|
||
Analyte |
Sample |
(ml) |
(mm mm) |
volume/solvent |
(m mm) |
Detection a |
(ng l 1) |
Reference |
|
Atrazine |
Water |
10 |
5 m Spherisorb |
Loop type |
RG: Deactivated (2 0.53) |
NPD |
3 |
|
98 |
|
|
|
(100 2) |
150 l |
PC: Carbowax 20M 0.4 m |
|
|
|
|
|
|
|
ODS |
methanol/water |
(3 0.32) |
|
|
|
|
|
|
|
|
(60 : 40) with 5% |
AC: 0.4 m Carbowax 20M |
|
|
|
|
|
|
|
|
1-propanol |
(40 0.32) |
|
|
|
|
Polycyclic |
Water |
0.098 |
5 m Krosil C18 |
On-column |
RG: Deactivated (5 0.53) |
FID |
n.s. |
|
99 |
aromatic |
|
|
(100 3.1) |
75 l ethyl |
AC: 0.17 m HP1 |
|
|
|
|
hydrocarbons |
|
|
SPE: 10 m PLRPS |
acetate |
(25 0.32) |
|
|
|
|
|
|
|
(10 2) |
|
|
|
|
|
|
Organo- |
Water |
2.5 |
Thick extraction |
On-column |
RG: Deactivated (5 0.32) |
NPD |
10 |
– 100 |
91 |
phosphorus |
|
|
(15 4.2) discs |
70 l ethyl |
PC: 0.5 m DB-5 (3 0.32) |
|
|
|
|
pesticides |
|
|
90% C18, 10% |
acetate |
AC: 0.5 m DB-5 |
|
|
|
|
|
|
|
XAD |
|
(25 0.32) |
|
|
|
|
Organo- |
Water |
10 |
10 m PLRP-S |
On-column |
RG: Deactivated (5 0.32) |
FID |
100 |
|
90 |
phosphorus |
|
|
(10 2) |
100 l ethyl |
AC: 0.14 m DB-1 |
NPD |
0.4 |
– 6.0 |
|
and organo- |
|
|
Drying cartridge |
acetate |
(15 0.32) |
FPD |
1 |
– 10 |
|
sulfur |
|
|
|
|
|
|
|
|
|
pesticides |
|
|
|
|
|
|
|
|
|
Organo- |
Water |
2 |
15 – 25 m PLRP-S |
Loop type 500 l |
RG: Deactivated (10 0.53) |
NPD |
10 |
|
95 |
phosphorus |
|
|
(10 2) |
methyl-t-butyl |
AC: 0.2 m CP Si1 – 19 CB |
|
|
|
|
pesticides |
|
|
|
ether with 10% |
(50 0.32) |
|
|
|
|
|
|
|
|
ethyl acetate; |
|
|
|
|
|
|
|
|
|
co-solvent, |
|
|
|
|
|
|
|
|
|
100 l n-decane |
|
|
|
|
|
Alkylated, |
Urban air |
20 l of |
5 m Silica |
On-column |
RG: Deactivated (10 0.53) |
MS |
n.q. |
|
97 |
oxygenated |
particulate |
hexane |
(100 2) |
loop type 350 l |
PC: not given |
FID |
n.s. |
|
|
and nitrated |
extracts |
extrate |
|
pentane |
AC: 0.5 m DMS |
|
|
|
|
polycyclic |
|
from |
|
dichloromethane |
(25 0.32) |
|
|
|
|
aromatic |
|
SFE |
|
(50-50) |
|
|
|
|
|
compounds |
|
|
|
|
|
|
|
|
|

Table 13.2 (continued )
|
|
Sample |
|
Interface/ |
|
|
LOD b |
|
|
|
|
volume |
LC column |
Transfer |
GC system |
|
|
||
Analyte |
Sample |
(ml) |
(mm mm) |
volume/solvent |
(m mm) |
Detection a |
(ng l 1) |
Reference |
|
|
|
|
|
|
|
|
|
|
|
Pesticides |
Water |
10 |
15 – 25 m |
Loop type |
RG: Deactivated (5 0.53) |
MS/MS |
0.01 |
– 0.50 |
100 |
|
|
|
PLRPS |
100 l ethyl |
PC: 0.25 m HP-5MS |
|
|
|
|
|
|
|
(10 2) |
acetate; |
(1 0.25) |
|
|
|
|
|
|
|
|
co-solvent, |
AC: 0.25 m HP-5MS |
|
|
|
|
|
|
|
|
50 l ethyl |
(30 0.25) |
|
|
|
|
|
|
|
|
acetate |
|
|
|
|
|
Phthalates |
Water |
10 |
5 m C18 |
PTV 2cm bed |
PC: 1 m PS-255 (1 0.53) |
MS |
5 |
– 10 |
96 |
|
|
|
(10 3) |
Carbofrit |
AC: 0.5 m PS-255 (20 |
|
|
|
|
|
|
|
|
720 l methanol/ |
0.25) |
|
|
|
|
|
|
|
|
water (85 : 15) |
|
|
|
|
|
Pesticides |
Water |
10 |
20 m PLRP-S |
On-column |
RG: Deactivated (5 0.53) |
MS |
2 |
– 20 |
92 |
|
|
|
(10 2) |
100 l ethyl |
PC: 0.25 m HP-5MS |
|
|
|
|
|
|
|
|
acetate |
(2 0.25) |
|
|
|
|
|
|
|
|
|
AC: 0.25 m HP5MS |
|
|
|
|
|
|
|
|
|
(3 0.25) |
|
|
|
|
Alkyl-, |
Water |
10 |
15 – 25 m |
Loop type |
RG: Deactivated (5 0.53) |
MS |
1 |
– 25 |
101 |
chloro-, and |
|
|
PLRP-S |
100 l ethyl |
PC: 0.52 m HPUltra |
|
|
|
|
mononitro- |
|
|
(10 2) |
acetate |
(3 0.32) |
|
|
|
|
phenols |
|
|
|
|
AC: 0.52 m HPUltra 2 |
|
|
|
|
|
|
|
|
|
(25 0.32) |
|
|
|
|
s-triazines |
Water |
10 |
Immunoaffinity |
On-column |
RG: Deactivated (0.2 0.1) |
|
|
|
|
|
|
|
(10 3) |
100 l ethyl |
PC: 0.25 m HP-5MS |
FID |
15 |
|
|
|
|
|
sorbent and |
acetate |
(1.5 0.25) |
NPD |
1.5 |
|
94 |
|
|
|
20 m PLRP-S |
|
AC: 0.25 m HP-5MS |
|
|
|
|
|
|
|
(10 2) |
|
(25 0.25) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
aNPD, nitrogen – phosphorus detector; FID, flame-ionization detector; MS, mass spectrometer.
bn.q., not quantified; n.s., not specified.

Multidimensional Chromatography in Environmental Analysis |
365 |
Figure 13.16 LC separation of urban air particulate extract (a), along with the GC/FID chromatogram (b) of an oxy-PAC fraction (transferred via a loop-type interface). Reprinted from
Environmental Science and Technology, 29, A. C. Lewis et al., ‘On-line coupled LC – GC – ITD/MS for the identification of alkylated, oxygenated and nitrated polycyclic aromatic compounds in urban air particulate extracts’, pp. 1977 – 1981, copyright 1995, with permission from the American Chemical Society.
in water by use of the vaporizer/precolumn solvent split gas discharge interface (96). The fraction from the LC column, eluted by the mobile phase consisting of water/methanol (15 : 85, v/v), is directly inserted into the PTV interface. The eluent is vaporized in a chamber kept at a high temperature which depends on the transfer flow. The transfer line contained packing material, i.e. Carbofrit, for smooth evaporation to occur. This was situated at the top of the properly heated area in order to prevent the transfer line from entering too far into the hot area. The solvent vapours were largely discharged through an early vapour exit, driven by the flow of carrier gas. Solvent/solute separation occurred in the precolumn. An uncoated retention gap was not necessary since water or water/methanol mixtures do not wet precolumn
366 |
Multidimensional Chromatography |
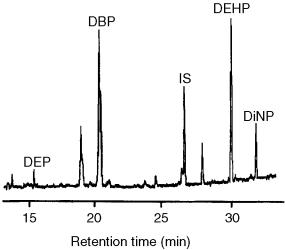
Figure 13.17 LC– GC– MS(EI) chromatogram of a treated drinking water containing 55 and 40 ng l 1, respectively, of DBP and DEHP. Reprinted from Journal of High Resolution Chromatography, 20, T. Hyötyläinen et al., ‘Reversed phase HPLC coupled on-line to GC by the vaporizer/precolumn solvent split gas discharge interface; analysis of phthalates in water’, pp. 410 – 416, 1997, with permission from Wiley-VCH.
surfaces and solvent trapping can not be used to improve the retention of volatile solutes. The experimental conditions for this analysis are shown in Table 13.2. This study has demonstrated that column temperature during transfer is a very important parameter which depends to a large extent on the volatility of the compounds and the composition of the mobile phase. However, problems with volatile solutes can still be significant.
For the LC separation, 10 ml of sample was injected through a loop. The LC flowrate was 1000 l min 1 and at the end of the enrichment process this was reduced to 100 l min 1. The mobile phase composition was optimized to eliminate matrix polar compounds and elute the phthalates in a small fraction.
Figure 13.17 shows the LC – GC – MS chromatogram of treated drinking water containing 55 and 40 ng l 1 of di-n-butylpthalate (DBP) and di(2-ethylhexyl)phtha- late (DEHP), respectively. The PTV system therefore allows the LC eluent to be injected directly and no change in the composition is needed.
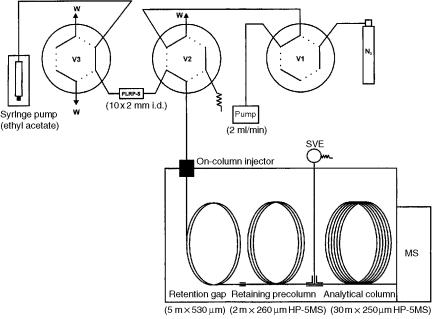
However, the most frequently used system is trace enrichment in a short LC column or SPE precolumn. The column is usually filled with C18 or PLRP-S and dried with nitrogen prior to elution. The analytes are eluted with an organic solvent, usually ethyl acetate, which is injected into the gas chromatograph through an oncolumn interface by a retention gap. In the chromatograph there is also a retaining precolumn, to minimize losses of the most volatile compounds, an analytical column and, between the precolumn and the analytical column, a solvent vapour exit (SVE) to eliminate the vapour (Figure 13.18).
Multidimensional Chromatography in Environmental Analysis |
367 |
Figure 13.18 Schematic diagram of the set-up used for on-line SPE– GC– MS.
This set-up, or a very similar one, has been used to determine different group of pollutants in environmental waters (45, 83, 93). For example, with 10 ml of sample the limits of detection of a group of pesticides were between 2 and 20 ng l 1 (92) in tap and river water, with this system being fully automated. Figure 13.19 shows the chromatograms obtained by on-line SPE – GC – MS under selected ion-monitoring conditions of 10 ml of tap water spiked with pesticides at levels of 0.1 g l 1 (92).
One disadvantage of this system in routine analysis is the long time required for the drying step. An alternative system has therefore been described (90, 102) and used to analyse water samples. This includes a drying precolumn after the SPE precolumn and before the entrance to the GC unit. Silica, copper sulfate and sodium sulfate cartridges were tested, with the results depending on the types of pesticides studied. One disadvantage of this system is the need to dry the precolumn after each sample or after a few samples. However, with an additional switching valve the precolumn can be regenerated by simultaneous heating and purging with a moisturefree gas during the GC run and this increases the sample throughput considerably. This system has been successfully applied to determine different types of pollutants (83).
The low selectivity of the SPE columns currently in use can be increased with more selective sorbents such as the immunosorbents, which have been quite extensively used in SPE – LC (72). Immunoaffinity-based solid-phase extraction (IASPE) sorbents have also been used in coupled gas chromatography for determining

368 |
Multidimensional Chromatography |
Figure 13.19 Chromatograms obtained by on-line SPE – GC – MS(SIM) of: (a) 10 ml of tap water spiked with pesticides at levels of 0.1 ng l 1; (b) 10 ml of a sample of unspiked tap water. Peak identification for (a) is as follows: 1, molinate; 2, -HCH; 3, dimethoate; 4, simazine; 5, atrazine; 6, -HCH; 7, -HCH; 8, heptachlor; 9, ametryn; 10, prometryn; 11, fenitrothion; 12, aldrin; 13, malathion; 14, endo-heptachlor; 15, -endosulfan; 16, tetrachlorvinphos; 17, dieldrin. Reprinted from Journal of Chromatography, A 818, E. Pocurull et al., ‘On-line coupling of solid-phase extraction to gas chromatography with mass spectrometric detection to determine pesticides in water’, pp. 85 – 93, copyright 1998, with permission from Elsevier Science.
micropollutants (94, 103) although such coupling is rather difficult to achieve. In order to remove these difficulties, the cartridge that contained the immobilized antibodies was coupled to the GC unit via a reverse-phase cartridge (PLRP-S) in the determination of s-triazines (94). After enrichment of the analytes on the immunoaffinity cartridge, they were desorbed and recollected on the PLRP-S cartridge by using an acidic buffer. The PLRP-S cartridge was then cleaned and dried with nitrogen. Finally, the desorption and transfer steps were carried out with ethyl acetate via an on-column interface.
The high selectivity of this system is demonstrated in Figure 13.20, which shows that a non-selective FID could be used to detect triazines in complex matrices, and that with 10 ml of sample the detection limits were 15 – 25 ng l 1. The experimental conditions are shown in Table 13.2.
The on-column interface is the one which is most often used in LC – GC of aqueous samples because it can be applied to a wider range of compounds.The loop-type interface is limited for determining volatile compounds that are volatilized together with the solvent and not retained in the retention gap. Several attempts at solving this problem have been made. One option is to add a co-solvent which enters the retention gap before the analytes and thus forms a co-solvent film in front of the eluate.
