
Multidimensional Chromatography
.pdf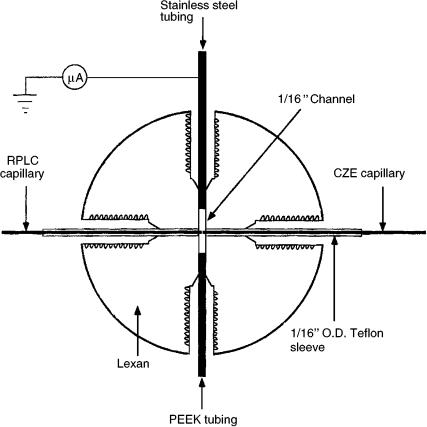
Multidimensional Electrodriven Separations |
211 |
Figure 9.11 Schematic illustration of the transparent interface used to link the HPLC capillary to the CZE capillary. Reprinted from Analytical Chemistry, 69, T. F. Hooker and J. W. Jorgenson, ‘A transparent flow gating interface for the coupling of microcolumn LC with CZE in a comprehensive two-dimensional system’, pp 4134 – 4142, copyright 1997, with permission from the American Chemical Society.
problem in the previous -HPLC – CZE system, but with the transparent interface this was much easier. This -HPLC – CZE separation system yielded high peak capacities, producing over 400 resolved peaks from biological samples (27).
9.13 ONLINE REVERSE PHASE HIGH PERFORMANCE LIQUID CHROMATOGRAPHY– CAPILLARY ZONE ELECTROPHORESIS – MASS SPECTROMETRY
Mass spectrometry (MS) is increasingly being combined with reverse phase HPLC or CZE in order to add an additional dimension to the data that a traditional detection system would not provide. A two-dimensional LC – CZE system with mass

212 |
Multidimensional Chromatography |
spectrometric detection was designed by Jorgenson and co-workers in 1997. In the coupling of the CZE capillary to the MS detector, a new microelectrospray needle was developed. Figure 9.12 shows a diagram of the silica sheath electrospray needle specially designed for this instrument. This needle produced high ionization efficiency, low flow rates, and a sheath flow that enabled the CZE to operate near optimal conditions. A transverse flow gating interface was again used to couple the reverse phase HPLC column to the CZE column. The result of this separation system was the combination of the resolving power of reverse phase HPLC and CZE, with mass spectrometric detection, all within 15 min (28).
9.14 THE FUTURE OF MULTIDIMENSIONAL ELECTROKINETIC SEPARATIONS
Electrodriven separation techniques are destined to be included in many future multidimensional systems, as CE is increasingly accepted in the analytical laboratory. The combination of LC and CE should become easier as vendors work towards providing enhanced microscale pumps, injectors, and detectors (18). Detection is often a problem in capillary techniques due to the short path length that is inherent in the capillary. The work by Jorgenson’s group mainly involved fluorescence detection to overcome this limit in the sensitivity of detection, although UV–VIS would be less restrictive in the types of analytes detected. Increasingly sensitive detectors of many types will make the use of all kinds of capillary electrophoretic techniques more popular.
Data analysis is one aspect of multidimensional analyses that must be optimized in the future. The analysis of chromatographic data beyond one dimension is still exceedingly problematic, especially in the analyses of highly complex mixtures. Better software may need to be developed in order to analyze twoand three-dimen- sional peaks due to their complexity. Three-dimensional data is only useful today in terms of fingerprinting and often that even requires extensive data analysis. A great deal of research must still be carried out to make the interpretation and quantification of multidimensional data easier.
Figure 9.12 Schematic diagram of the silica sheath electrospray needle used to interface capillary zone electrophoresis with a mass spectrometer.
Multidimensional Electrodriven Separations |
213 |
9.15 CONCLUSIONS
Electrodriven techniques are useful as components in multidimensional separation systems due to their unique mechanisms of separation, high efficiency and speed. The work carried out by Jorgenson and co-workers has demonstrated the high efficiencies and peak capacities that are possible with comprehensive multidimensional electrodriven separations. The speed and efficiency of CZE makes it possibly the best technique to use for the final dimension in a liquid phase multidimensional separation. It can be envisaged that multidimensional electrodriven techniques will eventually be applied to the analysis of complex mixtures of all types. The peak capacities that can result from these techniques make them extraordinarily powerful tools. When the limitations of one-dimensional separations are finally realized, and the simplicity of multidimensional methods is enhanced, the use of multidimensional electrodriven separations may become more widespread.
REFERENCES
1.J. W. Jorgenson, ‘Overview of electrophoresis’, in New Directions in Electrophoretic Methods, Jorgenson, J. W. and M. Phillips (Eds), ACS Symposium Series 335, American Chemical Society, Washington, DC, pp. 1 – 19 (1987).
2.A. Tiselius, ‘Electrophoresis of serum globulin’, J. Biochem. 31: 313 – 317 (1937).
3.F. E. P. Mikkers, F. M. Everaerts and P. E. M. Th. Verheggen, ‘High-performance zone electrophoresis’, J. Chromatogr. 169: 11 – 20 (1979).
4.J. W. Jorgenson and K. DeArman Lukacs, ‘Zone electrophoresis in open-tubular glass capillaries’, Anal. Chem. 53: 1298 – 1302 (1981).
5.J. C. Giddings, Unified Separation Science, John Wiley & Sons, New York, pp. 126 – 128 (1991).
6.R. Consden, A. H. Gordon and A. J. P. Martin, ‘Qualitative analysis of proteins: partition chromatographic method using paper’, J. Biochem. 38: 224 – 232 (1944).
7.G. Haugaard and T. D. Kroner, ‘Partition chromatography of amino acids with applied voltage’, J. Am. Chem. Soc. 70: 2135 – 2137 (1948).
8.E. L. Durrum, ‘Two-dimensional electrophoresis and ionophoresis’, J. Colloid Sci. 6: 274 – 290 (1951).
9.K. Keck and U. Hagen, ‘Separation of the DNA [deoxyribonucleic acid] units on cellulose layers’, Biochim. Biophys. Acta. 87: 685 – 687 (1964).
10. P. H. O’Farrell, ‘High resolution two-dimensional electrophoresis of proteins’,
J. Biol. Chem. 250: 4007 – 4021 (1975).
11.W. G. Burton, K. D. Nugent, T. K. Slattery, B. R. Summers and L. R. Snyder, ‘Separation of proteins by reversed-phase high-performance liquid chromatography’, J. Chromatogr. 443: 363 – 379 (1988).
12.P. D. Grossman, J. C. Colburn, H. H. Lauer, R. G. Nielsen, R. M. Riggin, G. S. Sittampalam and E. C. Rickard, ‘Application of free-solution capillary electrophoresis to the analytical scale separation of proteins and peptides’, Anal. Chem. 61: 1186 – 1194 (1989).
13.H. Yamamoto, T. Manabe and T. Okuyama, ‘Gel permeation chromatography combined with capillary electrophoresis for microanalysis of proteins’, J. Chromatogr. 480: 277 – 283 (1989).
214 |
Multidimensional Chromatography |
14.H. Yamamoto, T. Manabe and T. Okuyama, ‘Apparatus for coupled high-performance liquid chromatography and capillary electrophoresis in the analysis of complex protein mixtures’, J. Chromatogr. 515: 659 – 666 (1990).
15.M. Castagnola, L. Cassiano, R. Rabino, D. V. Rossetti and F. Andreasi Bassi, ‘Peptide mapping through the coupling of capillary electrophoresis and high-performance liquid chromatography: map prediction of the tryptic digest of myoglobin’, J. Chromatogr. 572: 51 – 58 (1991).
16.S. Pálmarsdóttir and L. E. Edholm, ‘Enhancement of selectivity and concentration sensitivity in capillary zone electrophoresis by on-line coupling with column liquid chromatography and utilizing a double stacking procedure allowing for microliter injections’,
J.Chromatogr. 693: 131 – 143 (1995).
17.M. Strömqvist, ‘Peptide mapping using combinations of size-exclusion chromatography, reversed-phase chromatography and capillary electrophoresis’, J. Chromatogr. 667: 304 – 310 (1994).
18.T. F. Hooker, D. J. Jeffery and J. W. Jorgenson, ‘Two-dimensional separations in highperformance capillary electrophoresis, in High Performance Capillary Electrophoresis,
M.G. Khakedi (Ed.), John Wiley & Sons, New York, pp. 581 – 612 (1998).
19. M. M. Bushey and J. W. Jorgenson, ‘Automated instrumentation for comprehensive twodimensional high-performance liquid chromatography/capillary zone electrophoresis’, Anal. Chem. 62: 978 – 984 (1990).
20.J. P. Larmann-Jr, A. V. Lemmo, A. W. Moore and J. W. Jorgenson, ‘Two-dimensional separations of peptides and proteins by comprehensive liquid chromatography – capillary electrophoresis’, Electrophoresis 14: 439 – 447 (1993).
21.R. Weinberger, ‘The sixth annual Frederick conference on capillary electrophoresis’, Am. Lab. 28: 42 – 43 (1996).
22.A. V. Lemmo and J. W. Jorgenson, ‘Two-dimensional protein separation by microcolumn size-exclusion chromatography – capillary zone electrophoresis’, J. Chromatogr. 633: 213 – 220 (1993).
23.A. V. Lemmo and J. W. Jorgenson, ‘Transverse flow gating interface for the coupling of microcolumn LC with CZE in a comprehensive two-dimensional system’, Anal. Chem. 65: 1576 – 1581 (1993).
24.C. A. Monnig and J. W. Jorgenson, ‘On-column sample gating for high-speed capillary zone electrophoresis’, Anal. Chem. 63: 802 – 807 (1991).
25.A. W. Moore-Jr and J. W. Jorgenson, ‘Rapid comprehensive two-dimensional separations of peptides via RPLC-optically gated capillary zone electrophoresis’, Anal. Chem. 67: 3448 – 3455 (1995).
26.A. W. Moore, Jr and J. W. Jorgenson, ‘Comprehensive three-dimensional separation of peptides using size exclusion chromatography/reversed phase liquid chromatography/ optically gated capillary zone electrophoresis’, Anal. Chem. 67: 3456 – 3463 (1995).
27.T. F. Hooker and J. W. Jorgenson, ‘A transparent flow gating interface for the coupling of microcolumn LC with CZE in a comprehensive two-dimensional system’, Anal. Chem. 69: 4134 – 4142 (1997).
28.K. C. Lewis, G. J. Opiteck, J. W. Jorgenson and D. M. Sheeley, ‘Comprehensive online RPLC – CZE – MS of peptides’, J. Am. Soc. Mass Spectrom. 8: 495 – 500 (1997).
Multidimensional Chromatography
Edited by Luigi Mondello, Alastair C. Lewis and Keith D. Bartle Copyright © 2002 John Wiley & Sons Ltd ISBNs: 0-471-98869-3 (Hardback); 0-470-84577-5 (Electronic)
Part 2
Applications

Multidimensional Chromatography
Edited by Luigi Mondello, Alastair C. Lewis and Keith D. Bartle
Copyright © 2002 John Wiley & Sons Ltd
ISBNs: 0-471-98869-3 (Hardback); 0-470-84577-5 (Electronic)
10Multidimensional Chromatography: Foods, Flavours and Fragrances Applications
G. DUGO, P. DUGO and L. MONDELLO
Università di Messina, Messina, Italy
10.1INTRODUCTION
Chromatography is the best technique for the separation of complex mixtures. Frequently, samples to be analysed are very complex, so the analyst has to choose more and more sophisticated techniques. Multidimensional separations, off-line and recently on-line, have been used for the analysis of such complex samples.
Food, flavour and fragrance products are a good example of natural complex mixtures. The analysis of these matrices may be carried out to:
•determine the qualitative and/or quantitative composition of a specific class of components;
•control the quality and the authenticity of the product;
•detect the presence of adulteration or contamination.
Sometimes, the monodimensional separation cannot be sufficient to resolve all of the components of interest. Problems of peak overlapping may occur, and a preseparation of the sample is often necessary. This pre-separation has the aim of reducing the complexity of the original sample matrix, by separating a simpler fraction than the original matrix. The fraction should contain the same amount of the analyte as in the whole sample, ready for analysis and free from substances that can interfere during the chromatographic analysis. Often, the preseparation is carried out off-line because it is easy to operate, although it can present many disadvantages, such as long separation times, the possibility of contamination or formation of artefacts, the difficulty of a quantitative recovery of the components of interest, etc. On the other hand, many on-line pre-separation methods have now been developed that have the advantages of greatly reducing the total analysis time, compared to classical off-line sample preparation techniques, to give a good recovery of the analytes with minimal chance for contamination. The disadvantage is that the equipment is significantly more complex and expensive than for monodimensional chromatography.
218 |
Multidimensional Chromatography |
10.2 MULTIDIMENSIONAL GAS CHROMATOGRAPHY (GC–GC OR MDGC)
A large number of the organic compounds in food and beverages are chiral molecules. In addition, a significant number of the additives, flavours, fragrances, pesticides and preservatives that are used in the food industry are also chiral materials.
The enantiomeric distribution can be very useful for identifying adulterated foods and beverages, for controlling and monitoring fermentation processes and products, and evaluating age and storage effects (1).
The enantiomeric distribution of the components of essential oils can provide information on the authenticity and quality of the oil, on the geographical origin and on their biogenesis (2).
GC using chiral columns coated with derivatized cyclodextrin is the analytical technique most frequently employed for the determination of the enantiomeric ratio of volatile compounds. Food products, as well as flavours and fragrances, are usually very complex matrices, so direct GC analysis of the enantiomeric ratio of certain components is usually difficult. Often, the components of interest are present in trace amounts and problems of peak overlap may occur. The literature reports many examples of the use of multidimensional gas chromatography with a combination of a non-chiral pre-column and a chiral analytical column for this type of analysis.
Mosandl and his co-workers (3–17) have carried out many research studies on the determination of the enantiomeric ratio of various components of food and beverages, as well as plant materials and essential oils. Using a SiChromat 2–8 doubleoven system with two independent temperature controls, two flame ionization detectors and a ‘live switching’ coupling piece, these workers have developed many applications of enantioselective MDGC employing heart-cutting technique from a non-chiral pre-separation column on to a chiral main column. In this way, direct chiral analysis is possible without any further clean-up or derivatization procedure. Table 10.1 summarizes some of these applications. As a typical example, Figure 10.1(a) shows the separation on a Carbowax 20M column of a dichloromethane extract of a ‘strawberry’ tea (18). As can be seen, the GC profile is very complex. Figure 10.1(b) shows the enantiomeric separation of 2,5-dimethyl-4-hydroxy-3[2H]- furanone, known as ‘pineapple ketone’, from the tea extract, transferred from the Carbowax 20M pre-column to a modified -cyclodextrin column. This analysis allowed the detection of the synthetic racemate of ‘pineapple ketone’ that was added to the tea to give the strawberry flavour.
For the enantioselective flavour analysis of components present in extremely low concentrations, a MDGC–MS method has been developed (19). An example of the application of this technique is the determination of theaspiranes and theaspirones in fruits. These compounds are potent flavour compounds which are widely used in the flavours industry. Figure 10.2 shows the MDGC–MS chromatogram obtained by using multiple ion detection (MID) differentiation between the enantiomers of theaspiranes in an aglycone fraction from purple passion fruit. In fact, using the MID technique, interfering peaks are easily removed and the detection limit is lowered.

Table 10.1 Applications of MDGC reported by Mosandl and his co-workers. These were developed by using a Siemens SiChromat 2 double-oven system with two independent temperature controls, two flame-ionization detectors and a ‘live switching’ coupling piece, employing the heartcutting technique
Application |
Pre-column |
Main column |
Reference |
|
|
|
|
Enantiomeric distribution of -lactone |
Fused silica retention gap (10 m |
Glass capillary column (38 m 0.2 mm |
3 |
homologues from different apricot |
0.25 mm i.d.) coupled to a DB-1701 |
i.d.) coated with heptakis (3-O- |
|
cultivars. Identification of dihydro- |
column (15 m 0.25 mm i.d.; 1 m |
acetyl-2,6-di-O-pentyl)- - |
|
actinidiolide (co-eluted with -C11 on |
1 mfilm thickness) |
cyclodextrin |
|
DB-1701) |
|
|
|
Determination of chiral- -lactones from raw flavour extract of strawberries and other- fruit-containing foods and beverages
Determination of chiral -pinene, -pinene and limonene in essential oils and plant extracts
Fused silica retention gap (10 m 0.25 mm i.d.) coupled to a DB-1701 column (15 m 0.25 mm i.d; 1 m film thickness)
Fused silica SupelcowaxTM 10 capillary column (60 m 0.32 mm i.d; 0.25 m film thickness)
Glass capillary column (38 m 0.2 mm |
4 |
i.d.) coated with heptakis (3-O- |
|
acetyl-2,6-di-O-pentyl)- - |
|
cyclodextrin |
|
Glass capillary column (47 m 0.23 mm |
5 |
i.d.) coated with heptakis |
|
(2,3,6-tri-O-methyl)- -cyclodextrin |
|
(10% in OV-1701-vinyl) |
|
Stereoanalysis of 2-alkyl-branched acids, esters and alcohols in apple aroma concentrate
Stereoanalysis of 2-methylbutanoate from apples and pineapples
Determination of enantiomeric distribution of some secondary alcohols and their acetates from banana
Restriction capillary (25 m 0.23 mm i.d.) coupled to a glass capillary column, (25 m 0.32 mm i.d.) coated with a 18.8% solution of PS-255 and 1.5% dicumyl peroxide.
Duran glass capillary (25 m 0.23 mm i.d.) coupled to a Superox 0.6 (0.25 m) glass capillary column
(27 m 0.32 mm i.d.)
Fused silica SupelcowaxTM 10 capillary column (60 m 0.32 mm i.d.;
0.25 m film thickness)
Glass capillary column (38 m 0.23 mm |
6 |
i.d.) coated with heptakis (2,3,6- |
|
tri-O-ethyl)- -cyclodextrin (33% in |
|
OV-1701-vinyl) |
|
Glass capillary column (38 m 0.23 mm |
7 |
i.d.) coated with heptakis (2,3,6- |
|
tri-O-ethyl)- -cyclodextrin (33% |
|
in OV-1701-vinyl) |
|
Glass capillary column (47 m 0.23 mm |
8 |
i.d.) coated with heptakis (2,3,6- |
|
tri-O-methyl)- -cyclodextrin |
|
(10% in OV-1701-vinyl) |
|
Applications Fragrances and Flavours Foods,
219

Table 10.1 (continued )
Application |
Pre-column |
Main column |
Reference |
Determination of enantiomeric distribution |
Fused silica SupelcowaxTM 10 capillary |
Duranglas glass capillary column |
9 |
of monoterpenoids from geranium oil |
column, (60 m 0.32 mm i.d.; |
(26 m 0.23 mm i.d.) coated with |
|
|
0.25 m film thickness) |
heptakis (2,3-di-O-acetyl-6-O-tert |
|
|
|
butyldimethyl-1-silyl)- -cyclodextrin |
|
|
|
(50% in OV-1701 vinyl) |
|
Determination of enantiomeric distributionof the lactone flavour compounds of fruits
OV-1701 fused silica capillary column, (50 m 0.32 mm i.d., 0.25 m film thickness)
Glass capillary column (47 m 0.23 |
10 |
mm i.d.) coated with octakis (3-O-butiryl, 2,6-di-O-pentyl--cyclodextrin
Determination of enantiomeric distribution of chiral components of
Cymbopogon oil
Determination of the enantiomeric distribution of the chiral major compounds of buchu leaf oil
Determination of the enantiomeric distribution of 2-, 3-, and 4-alkyl- branched acids from Roman Chamomile and Parmesan cheese
Polyethylene glycol
Duranglas glass capillary column, (44 m 0.23 mm i.d.) coated with a 0.5 m film of OV-215 vinyl
Glass capillary column (30 m 0.32 mm i.d.) coated with SE-52
(0.65 m film thickness)
Glass capillary column (47 m 0.23 |
11 |
mm i.d.) coated with heptakis |
|
(2,3-di-O-acetyl-6-O-tert- |
|
butyldimethy-l-1-silyl)- -cylodextrin |
|
(50% in OV-1701-vinyl) |
|
Duranglas capillary column (15 m |
12 |
0.23 mm i.d.) coated with heptakis |
|
(2,3-di-O-acetyl-6-O-tert- |
|
butyldimethy l-1-silyl)- -cyclodextrin |
|
(25%), octakis (2,3-di-O-acety l-6-O- |
|
tert-butyldimethylsilyl)- -cyclodextrin |
|
(25%) and OV-1701-vinyl (50%) |
|
Glass capillary column (30 m 0.23 mm |
13 |
i.d.) coated with 15% heptakis (2,3-di- |
|
O-methyl-6-O-tert butyldimethylsilyl)--cyclodextrin in PS-268 (0.23 m film thickness)
220
Chromatography Multidimensional

Determination of the enantiomeric distribution of 4-methyl-5-decanolide in white flowering aochids (Aerangis confusa)
Determination of the enantiomeric ratio of linalol and linalyl acetate in bergamot oil
Determination of the enantiomeric ratio of-pinene, -pinene and limonene in bergamot oil
Determination of the enantiomeric distribution of linalol, linalyl acetate and-terpineol in neroli and petitgrain oils
Determination of the enantiomeric distribution of -pinene, -pinene, limonene, terpinen-4-ol and nerolidol in neroli and petitgrain oils
Determination of the enantiomeric distribution of some chiral sulfurcontaining trace components of yellow passion fruit a
Duranglas glass capillary column (30 m 0.23 mm i.d.) coated with SE52 (0.63 m film thickness)
Fused silica SupelcowaxTM 10 capillary column (30 m 0.32 mm i.d.;
0.23 mm film thickness)
Duranglas glass capillary column (30 m 0.23 mm i.d.) coated with OV-215 (0.23 m film thickness)
Fused silica Supelcowax™ 10 capillary column (30 m 0.32 mm i.d.;
0.23 m film thickness)
Duranglas glass capillary column (30 m 0.23 mm i.d.) coated with OV-215 (0.23 m film thickness)
DB-210 fused silica capillary column (30 m 0.32 mm i.d.; film thickness 0.25 m)
Fused silica column (30 m 0.25 mm |
14 |
i.d.) coated with 15% of heptakis (2,3- |
|
di-O-methyl-6-O-tert-butyl- |
|
dimethylsilyl)- -cyclodextrin in |
|
PS-268 (0.25 m film thickness) |
|
Duranglas glass capillary column |
15 |
(26 m 0.23 mm) coated with |
|
heptakis (2,3-di-O-acetyl-6-O-tert- |
|
butyldimethylsily1)- -cyclodextrin |
|
(50% in OV-1701 vinyl) |
|
Duranglas glass capillary column |
15 |
(25 m 0.23 mm i.d.) coated with |
|
heptakis (2,3-di-O-methyl-6-O-tert- |
|
butyldimethylsilyl)- -cyclodextrin |
|
(50% in OV-1701 vinyl) |
|
Duranglas glass capillary column |
16 |
(26 m 0.23 mm) coated with |
|
heptakis (2,3-di-O-acetyl-6-O- |
|
tertbutyldimethylsilyl)- -cyclodextrin |
|
(50% in OV-1701 vinyl) |
|
Duranglas glass capillary column |
16 |
(25 m 0.23 mm i.d.) coated with |
|
heptakis (2,3-di-O-methyl-6-O-tert- |
|
butyldimethylsilyl)- -cyclodextrin |
|
(50% in OV-1701 vinyl) |
|
Fused silica column (30 m 0.32 mm |
17 |
i.d.; film thickness 0.32 m) coated |
|
with octakis (2,3-di-O-butyryl-6-O- tertbutyldimethylsilyl)- -cyclodextrin (50% in OV-1701 vinyl)
Applications Fragrances and Flavours Foods,
aApplication developed by using a Fisons GC 8000 chromatograph where the two columns were installed and coupled via a moving capillary stream switching (MCSS) system. The chromatograph was equiped with a flame-ionization detector on the MCSS system outlet and a Flame-photometric detector on the main column outlet, and a split/splitless injector.
221
